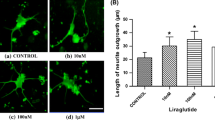
Abstract
Glucagon-like peptide-1 (GLP-1) is an insulinotropic peptide with neurotrophic properties, as assessed in animal cell models. Exendin-4, a GLP-1 analogue, has been recently approved for the treatment of type 2 diabetes mellitus. The aim of this study was to morphologically, structurally, and functionally characterize the differentiating actions of exendin-4 using a human neuronal cell model (i.e., SH-SY5Y cells). We found that exendin-4 increased the number of neurites paralleled by dramatic changes in intracellular actin and tubulin distribution. Electrophysiological analyses showed an increase in cell membrane surface and in stretch-activated-channels sensitivity, an increased conductance of Na+ channels and amplitude of Ca++ currents (T- and L-type), typical of a more mature neuronal phenotype. To our knowledge, this is the first demonstration that exendin-4 promotes neuronal differentiation in human cells. Noteworthy, our data support the claimed favorable role of exendin-4 against diabetic neuropathy as well as against different neurodegenerative diseases.









Similar content being viewed by others
References
Zhou J, Pineyro MA, Wang X, Doyle ME, Egan JM (2002) Exendin-4 differentiation of a human pancreatic duct cell line into endocrine cells: involvement of PDX-1 and HNF3beta transcription factors. J Cell Physiol 192:304–314
Ugleholdt R, Zhu X, Deacon CF, Ørskov C, Steiner DF, Holst JJ (2004) Impaired intestinal proglucagon processing in mice lacking prohormone convertase 1. Endocrinology 145:1349–1355
Doyle ME, Egan JM (2007) Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113:546–593
Jang HJ, Kokrashvili Z, Theodorakis MJ (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104:15069–15074
Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA (1996) Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 81:327–332
Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N, Tarui S (1989) Glucagonostatic and insulinotropic action of glucagons-like peptide I-(7–36)-amide. Diabetes 38:902–905
Estall JL, Drucker DJ (2006) Glucagon and glucagon-like peptide receptors as drug targets. Curr Pharm Des 12:1731–1750
Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, Baron AD (2005) Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 62:173–181
Thorens B (1992) Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 89:8641–8645
Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI (1993) Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 133:2861–2870
Moens K, Heimberg H, Flamez D, Huypens P, Quartier E, Ling Z, Pipeleers D, Gremlich S, Thorens B, Schuit F (1996) Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes 45:257–261
Ahren B (2004) GLP-1 and extra-islet effects. Horm Metab Res 36:842–845
Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP (1995) Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7:2294–2300
Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Møller M, Sheikh SP (1996) Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol 271:R848–R856
Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR (1996) A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72
Meeran K, O’Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA (1999) Bloom SR. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 140:244–250
Baggio LL, Huang Q, Brown TJ, Drucker DJ (2004) Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 127:546–558
Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR (2005) The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044:127–131
Wei Y, Mojsov S (1995) Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett 358:219–224
Perry T, Greig NH (2002) The glucagon-like peptides: a new genre in therapeutic targets for intervention in Alzheimer’s disease. J Alzheimers Dis 4:487–496
During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN (2003) Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9:1173–1179
Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH (2002) Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther 302:881–888
Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH (2003) Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res 72:603–612
Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH (2009) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA 106:1285–1290
Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH (2002) A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther 300:958–966
Påhlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T (1984) Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ 14:135–144
Toselli M, Masetto S, Rossi P, Taglietti V (1991) Characterization of a voltage-dependent calcium current in the human neuroblastoma cell line SH-SY5Y during differentiation. Eur J Neurosci 3:514–522
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Benvenuti S, Luciani P, Vannelli GB, Gelmini S, Franceschi E, Serio M, Peri A (2005) Estrogen and selective estrogen receptor modulators exert neuroprotective effects and stimulate the expression of selective Alzheimer’s disease indicator-1: a recently discovered antiapoptotic gene, in human neuroblast long-term cell cultures. J Clin Endocrinol Metab 90:1775–1782
Luciani P, Ferruzzi P, Arnaldi G, Crescioli C, Benvenuti S, Nesi G, Valeri A, Greeve I, Serio M, Mannelli M, Peri A (2004) Expression of the novel adrenocorticotropin-responsive gene selective Alzheimer’s disease indicator-1 in the normal adrenal cortex and in adrenocortical adenomas and carcinomas. J Clin Endocrinol Metab 89:1332–1339
Formigli L, Francini F, Tani A, Squecco R, Nosi D, Polidori L, Nistri S, Chiappino L, Cesati V, Pacini A, Perna AM, Orlandini GE, Zecchi Orlandini S, Bani D (2005) Morphofunctional integration between skeletal myoblasts and adult cardiomyocytes in coculture is favored by direct cell-cell contacts and relaxin treatment. Am J Physiol Cell Physiol 288:795–804
Formigli L, Sassoli C, Squecco R, Bini F, Martinesi M, Chellini F, Luciani G, Sbrana F, Zecchi-Orlandini S, Francini F, Meacci E (2009) Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J Cell Sci 122:1322–1333
Abemayor E, Sidell N (1989) Human neuroblastoma cell lines as models for the in vitro study of neoplastic and neuronal cell differentiation. Environ Health Perspect 80:3–15
Govek EE, Newey SE, Van Aelst L (2005) The role of the Rho GTPases in neuronal development. Genes Dev 19:1–49
Kim W, Egan JM (2008) The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60:470–512
Liu J, Zheng X, Yin F, Hu Y, Guo L, Deng X, Chen G, Jiajia J, Zhang H (2006) Neurotrophic property of geniposide for inducing the neuronal differentiation of PC12 cells. Int J Dev Neurosci 24:419–424
Nakaso K, Ito S, Nakashima K (2008) Caffeine activates the PI3 K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci Lett 432:146–150
Miloso M, Villa D, Crimi M, Galbiati S, Donzelli E, Nicolini G, Tredici G (2004) Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is ERK independent and PKC dependent. J Neurosci Res 75:241–252
Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393:805–809
Chua BT, Volbracht C, Tan KO, Li R, Yu VC, Li P (2003) Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol 5:1083–1089
Sbrana F, Sassoli C, Meacci E, Nosi D, Squecco R, Paternostro F, Tiribilli B, Zecchi-Orlandini S, Francini F, Formigli L (2008) Role for stress fiber contraction in surface tension development and stretch-activated channel regulation in C2C12 myoblasts. Am J Physiol Cell Physiol 295:C160–C172
Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galié M, Sbarbati A, Krampera M, Belluzzi O, Bonetti (2008) Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev 17:909–916
Erceg S, Laínez S, Ronaghi M, Stojkovic P, Pérez-Aragó MA, Moreno-Manzano V, Moreno-Palanques R, Planells-Cases R, Stojkovic M (2008) Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One 3:e2122
Martinez-Monedero R, Yi E, Oshima K, Glowatzki E, Edge AS (2008) Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol 68:669–684
Yamaguchi Y, Katoh H, Yasui H, Mori K, Negishi M (2001) RhoA inhibits the nerve growth factor-induced Rac1 activation through Rho-associated kinase-dependent pathway. J Biol Chem 276:18977–18983
Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S (2000) A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron 26:431–441
Dehmelt L, Smart FM, Ozer RS, Halpain S (2003) The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci 23:9479–9490
Shea TB, Beermann ML (1994) Respective roles of neurofilaments, microtubules, MAP1B, and Tau in neurite outgrowth and stabilization. Mol Biol Cell 5:863–875
Berneistein BW, Bamburg JR (1992) Actin in emerging neurites is recruited from a monomer pool. Mol Neurobiol 6:95–106
Henley J, Poo MM (2004) Guiding neuronal growth cones using Ca21 signals. Trends Cell Biol 14:320–330
D’Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C (2006) Role of L-type Ca2 + channels in neural stem progenitor cell differentiation. Eur J Neurosci 23:935–944
Fischer AJ, Omar G, Walton NA, Verrill TA, Unson CG (2005) Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J Neurosci 25:10157–10166
Ichetovkin I, Grant W, Condeelis J (2002) Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr Biol 12:79–84
Rosen K, Rak J, Leung T, Dean NM, Kerbel RS, Filmus J (2000) Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J Cell Biol 149:447–456
Posey SC, Bierer BE (1999) Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J Biol Chem 274:4259–4265
Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, Falchi M, Malorni W, Fais S (2000) CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J 19:5123–5134
Yang EJ, Yoon JH, Min DS, Chung KC (2004) LIM kinase 1 activates cAMP-responsive element-binding protein during the neuronal differentiation of immortalized hippocampal progenitor cells. J Biol Chem 279:8903–8910
Gillespie LN (2003) Regulation of axonal growth and guidance by the neurotrophin family of neurotrophic factors. Clin Exp Pharmacol Physiol 30:724–733
O’Neill K, Chen S, Brinton RD (2004) Impact of the selective estrogen receptor modulator, raloxifene, on neuronal survival and outgrowth following toxic insults associated with aging and Alzheimer’s disease. Exp Neurol 185:63–80
Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K (2004) Abeta(25–35)-induced memory impairment, axonal atrophy and synaptic loss are ameliorated by M1, a metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology 29:860–868
Acknowledgments
This study was supported by a grant from Ente Cassa di Risparmio di Firenze.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luciani, P., Deledda, C., Benvenuti, S. et al. Differentiating effects of the glucagon-like peptide-1 analogue exendin-4 in a human neuronal cell model. Cell. Mol. Life Sci. 67, 3711–3723 (2010). https://doi.org/10.1007/s00018-010-0398-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0398-3