Abstract
Background and aim
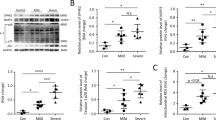
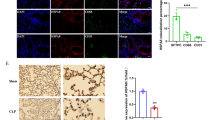
Sepsis-induced acute lung injury (ALI) is a complex and life-threatening condition lacking specific and efficient clinical treatments. Extracellular histones, identified as a novel type of damage-associated molecular patterns, have been implicated in the inflammatory process of ALI. However, further elucidation is needed regarding the precise mechanism through which extracellular histones induce inflammation. The aim of this study was to investigate whether extracellular histones can activate NLRP3 inflammasome-mediated inflammation in alveolar macrophages (AMs) by affecting TWIK2-dependent potassium efflux.
Methods and results
We conducted experiments using cecal ligation and puncture (CLP) C57BL/6 mice and extracellular histone-stimulated LPS-primed MH-S cells. The results demonstrated a significant increase in the levels of extracellular histones in the plasma and bronchoalveolar lavage fluid (BALF) of CLP mice. Furthermore, neutralizing extracellular histone mitigated lung injury and inflammation in CLP-induced ALI mice. In vitro studies confirmed that extracellular histones upregulated the expression of NLRP3 inflammasome activation-related proteins in MH-S cells, and this effect was dependent on increased potassium efflux mediated by the TWIK2 channel on the plasma membrane. Moreover, extracellular histones directly triggered a substantial influx of calcium, leading to increased Rab11 activity and facilitating the trafficking and location of TWIK2 to the plasma membrane.
Conclusion
These findings underscore the critical role of extracellular histone-induced upregulation of TWIK2 expression on the plasma membrane of alveolar macrophages (AMs). This upregulation leads to potassium efflux and subsequent activation of the NLRP3 inflammasome, ultimately exacerbating lung inflammation and injury during sepsis.










Similar content being viewed by others
Data availability
The data support the results of the present study are available from the corresponding author upon a reasonable request.
References
Beitler JR, Thompson BT, Baron RM, Bastarache JA, Denlinger LC, Esserman L, Gong MN, LaVange LM, Lewis RJ, Marshall JC, Martin TR, McAuley DF, Meyer NJ, Moss M, Reineck LA, Rubin E, Schmidt EP, Standiford TJ, Ware LB, Wong HR, Aggarwal NR, Calfee CS. Advancing precision medicine for acute respiratory distress syndrome. Lancet Respir Med. 2022;10(1):107–20. https://doi.org/10.1016/S2213-2600(21)00157-0.
Sheu CC, Gong MN, Zhai R, Chen F, Bajwa EK, Clardy PF, Gallagher DC, Thompson BT, Christiani DC. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest. 2010;138(3):559–67. https://doi.org/10.1378/chest.09-2933.
Xu H, Sheng S, Luo W, Xu X, Zhang Z. Acute respiratory distress syndrome heterogeneity and the septic ARDS subgroup. Front Immunol. 2023;14:1277161. https://doi.org/10.3389/fimmu.2023.1277161.
Cicchinelli S, Pignataro G, Gemma S, Piccioni A, Picozzi D, Ojetti V, Franceschi F, Candelli M. PAMPs and DAMPs in sepsis: a review of their molecular features and potential clinical implications. Int J Mol Sci. 2024;25(2):962. https://doi.org/10.3390/ijms25020962.
de Vries F, Huckriede J, Wichapong K, Reutelingsperger C, Nicolaes GAF. The role of extracellular histones in COVID-19. J Intern Med. 2023;293:275–92. https://doi.org/10.1111/joim.13585.
Ligi D, Lo Sasso B, Giglio RV, Maniscalco R, DellaFranca C, Agnello L, Ciaccio M, Mannello F. Circulating histones contribute to monocyte and MDW alterations as common mediators in classical and COVID-19 sepsis. Crit Care. 2022;26:260. https://doi.org/10.1186/s13054-022-04138-2.
Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. https://doi.org/10.1038/nm.2053.
Lv X, Wen T, Song J, Xie D, Wu L, Jiang X, Jiang P, Wen Z. Extracellular histones are clinically relevant mediators in the pathogenesis of acute respiratory distress syndrome. Respir Res. 2017;18:165. https://doi.org/10.1186/s12931-017-0651-5.
Zhang Y, Wen Z, Guan L, Jiang P, Gu T, Zhao J, Lv X, Wen T. Extracellular histones play an inflammatory role in acid aspiration-induced acute respiratory distress syndrome. Anesthesiology. 2015;122:127–39. https://doi.org/10.1097/ALN.0000000000000429.
Jiang P, Jin Y, Sun M, Jiang X, Yang J, Lv X, Wen Z. Extracellular histones aggravate inflammation in ARDS by promoting alveolar macrophage pyroptosis. Mol Immunol. 2021;135:53–61. https://doi.org/10.1016/j.molimm.2021.04.002.
Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192(12):5974–83. https://doi.org/10.4049/jimmunol.1400368.
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606. https://doi.org/10.1038/nrd.2018.97.
Fu J, Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol. 2023;41:301–16. https://doi.org/10.1146/annurev-immunol-081022-021207.
Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–53. https://doi.org/10.1016/j.immuni.2013.05.016.
Rivers-Auty J, Brough D. Potassium efflux fires the canon: potassium efflux as a common trigger for canonical and noncanonical NLRP3 pathways. Eur J Immunol. 2015;45:275827–61. https://doi.org/10.1002/eji.201545958.
Xu J, Núñez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. 2023;48:331–44. https://doi.org/10.1016/j.tibs.2022.10.002.
Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. https://doi.org/10.1152/physrev.00029.2009.
O’Kelly I. Endocytosis as a mode to regulate functional expression of two-pore domain potassium (K2P) channels. Pflugers Arch. 2015;467:1133–42. https://doi.org/10.1007/s00424-014-1641-9.
Nguyen NH, Brodsky JL. The cellular pathways that maintain the quality control and transport of diverse potassium channels. Biochim Biophys Acta Gene Regul Mech. 2023;1866: 194908. https://doi.org/10.1016/j.bbagrm.2023.194908.
Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M, Mittal M, Hong Z, Kanneganti TD, Rehman J, Malik AB. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity. 2018;49:56-65.e4. https://doi.org/10.1016/j.immuni.2018.04.032.
Drinkall S, Lawrence CB, Ossola B, Russell S, Bender C, Brice NB, Dawson LA, Harte M, Brough D. The two pore potassium channel THIK-1 regulates NLRP3 inflammasome activation. Glia. 2022;70:1301–16. https://doi.org/10.1002/glia.24174.
Busch CJ, Favret J, Geirsdóttir L, Molawi K, Sieweke MH. Isolation and long-term cultivation of mouse alveolar macrophages. Bio Protoc. 2019;9(14): e3302. https://doi.org/10.21769/BioProtoc.3302.
Kim SK, Joe Y, Chen Y, Ryu J, Lee JH, Cho GJ, Ryter SW, Chung HT. Carbon monoxide decreases interleukin-1β levels in the lung through the induction of pyrin. Cell Mol Immunol. 2017;14:349–59. https://doi.org/10.1038/cmi.2015.79.
Ding X, Yang DR, Xia L, Chen B, Yu S, Niu Y, Wang M, Li G, Chang C. Targeting TR4 nuclear receptor suppresses prostate cancer invasion via reduction of infiltrating macrophages with alteration of the TIMP-1/MMP2/MMP9 signals. Mol Cancer. 2015;14:16. https://doi.org/10.1186/s12943-014-0281-1.
Evavold CL, Hafner-Bratkovič I, Devant P, D’Andrea JM, Ngwa EM, Boršić E, Doench JG, LaFleur MW, Sharpe AH, Thiagarajah JR, Kagan JC. Control of gasdermin D oligomerization and pyroptosis by the ragulator-Rag-mTORC1 pathway. Cell. 2021;184:4495–511. https://doi.org/10.1016/j.cell.2021.06.028.
Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Crit Rev Clin Lab Sci. 2009;46:1–24. https://doi.org/10.1080/10408360802485875.
Holdenrieder S, Stieber P, Bodenmüller H, Busch M, Von Pawel J, Schalhorn A, Nagel D, Seidel D. Circulating nucleosomes in serum. Ann N Y Acad Sci. 2001;945:93–102. https://doi.org/10.1111/j.1749-6632.2001.tb03869.x.
Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, Standiford TJ, Ward PA. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27(12):5010–21. https://doi.org/10.1096/fj.13-236380.
Wu XY, Lv JY, Zhang SQ, Yi X, Xu ZW, Zhi YX, Zhao BX, Pang JX, Yung KKL, Liu SW, Zhou PZ. ML365 inhibits TWIK2 channel to block ATP-induced NLRP3 inflammasome. Acta Pharmacol Sin. 2022;43:992–1000. https://doi.org/10.1038/s41401-021-00739-9.
Borchers AC, Langemeyer L, Ungermann C. Who’s in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond. J Cell Biol. 2021;220: e202105120. https://doi.org/10.1083/jcb.202105120.
Homma Y, Hiragi S, Fukuda M. Rab family of small GTPases: an updated view on their regulation and functions. FEBS J. 2021;288:36–55. https://doi.org/10.1111/febs.15453.
Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–49. https://doi.org/10.1152/physrev.00059.2009.
Welz T, Wellbourne-Wood J, Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 2014;24:407–15. https://doi.org/10.1016/j.tcb.2014.02.004.
Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu M, Schwarzenberger C, Hohenstein B, Hugo C, Uhl B, Reichel CA, Krombach F, Monestier M, Liapis H, Moreth K, Schaefer L, Anders HJ. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23:1375–88. https://doi.org/10.1681/ASN.2011111077.
Lai JJ, Cruz FM, Rock KL. Immune sensing of cell death through recognition of histone sequences by C-type lectin-receptor-2d causes inflammation and tissue injury. Immunity. 2020;52:123–35. https://doi.org/10.1016/j.immuni.2019.11.013.
Wilson AS, Randall KL, Pettitt JA, Ellyard JI, Blumenthal A, Enders A, Quah BJ, Bopp T, Parish CR, Brüstle A. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat Commun. 2022;13:528. https://doi.org/10.1038/s41467-022-28172-4.
Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W, Wang G, Toh CH. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–9. https://doi.org/10.1164/rccm.201206-1037OC.
Hsieh IN, Deluna X, White MR, Hartshorn KL. Histone H4 directly stimulates neutrophil activation through membrane permeabilization. J Leukoc Biol. 2021;109:763–75. https://doi.org/10.1002/JLB.3A0620-342R.
Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, Winter J, Adrover JM, Santos GS, Froese A, Lemnitzer P, Ortega-Gómez A, Chevre R, Marschner J, Schumski A, Winter C, Perez-Olivares L, Pan C, Paulin N, Schoufour T, Hartwig H, González-Ramos S, Kamp F, Megens RTA, Mowen KA, Gunzer M, Maegdefessel L, Hackeng T, Lutgens E, Daemen M, von Blume J, Anders HJ, Nikolaev VO, Pellequer JL, Weber C, Hidalgo A, Nicolaes GAF, Wong GCL, Soehnlein O. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019;569:236–40. https://doi.org/10.1038/s41586-019-1167-6.
Jung J, Lee LE, Kim H, Kim JE, Jang SH, Roh JS, Lee B, Robinson WH, Sohn DH, Pyun JC, Song JJ. Extracellular histones aggravate autoimmune arthritis by lytic cell death. Front Immunol. 2022;13: 961197. https://doi.org/10.3389/fimmu.2022.961197.
Walker SA, Cullen PJ, Taylor JA, Lockyer PJ. Control of Ras cycling by Ca2+. FEBS Lett. 2003;546:6–10. https://doi.org/10.1016/s0014-5793(03)00412-5.
Kosuru R, Chrzanowska M. Integration of Rap1 and calcium signaling. Int J Mol Sci. 2020;21:1616. https://doi.org/10.3390/ijms21051616.
Miteva KT, Pedicini L, Wilson LA, Jayasinghe I, Slip RG, Marszalek K, Gaunt HJ, Bartoli F, Deivasigamani S, Sobradillo D, Beech DJ, McKeown L. Rab46 integrates Ca2+ and histamine signaling to regulate selective cargo release from Weibel-Palade bodies. J Cell Biol. 2019;218:2232–46. https://doi.org/10.1083/jcb.201810118.
Cook AA, Deng W, Ren J, Li R, Sondek J, Bergmeier W. Calcium-induced structural rearrangements release autoinhibition in the Rap-GEF CalDAG-GEFI. J Biol Chem. 2018;293:8521–9. https://doi.org/10.1074/jbc.RA118.002712.
Guidetti GF, Manganaro D, Consonni A, Canobbio I, Balduini C, Torti M. Phosphorylation of the guanine-nucleotide-exchange factor CalDAG-GEFI by protein kinase a regulates Ca(2+)-dependent activation of platelet Rap1b GTPase. Biochem J. 2013;453:115–23. https://doi.org/10.1042/BJ20130131.
Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–43. https://doi.org/10.1111/j.1600-0854.2004.00257.x.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. https://doi.org/10.1038/nrm1155.
Hsieh PC, Wu YK, Yang MC, Su WL, Kuo CY, Lan CC. Deciphering the role of damage-associated molecular patterns and inflammatory responses in acute lung injury. Life Sci. 2022;305: 120782. https://doi.org/10.1016/j.lfs.2022.120782.
Jaimes F, De La Rosa G, Morales C, Fortich F, Arango C, Aguirre D, Muñoz A. Unfractioned heparin for treatment of sepsis: a randomized clinical trial (the HETRASE study). Crit Care Med. 2009;37:1185–96. https://doi.org/10.1097/CCM.0b013e31819c06bc.
Yalcinkaya M, Fotakis P, Liu W, Endo-Umeda K, Dou H, Abramowicz S, Xiao T, Libby P, Wang N, Tall AR, Westerterp M. Cholesterol accumulation in macrophages drives NETosis in atherosclerotic plaques via IL-1β secretion. Cardiovasc Res. 2023;119:969–81. https://doi.org/10.1093/cvr/cvac189.
Rendeiro AF, Ravichandran H, Bram Y, Chandar V, Kim J, Meydan C, Park J, Foox J, Hether T, Warren S, Kim Y, Reeves J, Salvatore S, Mason CE, Swanson EC, Borczuk AC, Elemento O, Schwartz RE. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021;593:564–9. https://doi.org/10.1038/s41586-021-03475-6.
Liu Y, Xiao J, Cai J, Li R, Sui X, Zhang J, Lu T, Chen H, Chen G, Li H, Jiang C, Zhao X, Xiao C, Lei Y, Yao J, Lv G, Liang J, Zhang Y, Yang JR, Zheng J, Yang Y. Single-cell immune profiling of mouse liver aging reveals Cxcl2+ macrophages recruit neutrophils to aggravate liver injury. Hepatology. 2024;79:589–605. https://doi.org/10.1097/HEP.0000000000000590.
Aegerter H, Lambrecht BN, Jakubzick CV. Biology of lung macrophages in health and disease. Immunity. 2022;55:1564–80. https://doi.org/10.1016/j.immuni.2022.08.010.
Dang W, Tao Y, Xu X, Zhao H, Zou L, Li Y. The role of lung macrophages in acute respiratory distress syndrome. Inflamm Res. 2022;71:1417–32. https://doi.org/10.1007/s00011-022-01645-4.
Wang Y, Wang C, He Q, Chen G, Yu J, Cang J, Zhong M. Inhibition of sphingosine-1-phosphate receptor 3 suppresses ATP-induced NLRP3 inflammasome activation in macrophages via TWIK2-mediated potassium efflux. Front Immunol. 2023;14:1090202. https://doi.org/10.3389/fimmu.2023.1090202.
Huang LS, Anas M, Xu J, Zhou B, Toth PT, Krishnan Y, Di A, Malik AB. Endosomal trafficking of two-pore K+ efflux channel TWIK2 to plasmalemma mediates NLRP3 inflammasome activation and inflammatory injury. Elife. 2023;12: e83842. https://doi.org/10.7554/eLife.83842.
Bobak N, Feliciangeli S, Chen CC, Ben Soussia I, Bittner S, Pagnotta S, Ruck T, Biel M, Wahl-Schott C, Grimm C, Meuth SG, Lesage F. Recombinant tandem of pore-domains in a weakly inward rectifying K+ channel 2 (TWIK2) forms active lysosomal channels. Sci Rep. 2017;7:649. https://doi.org/10.1038/s41598-017-00640-8.
Jiang C, Liu Z, Hu R, Bo L, Minshall RD, Malik AB, Hu G. Inactivation of Rab11a GTPase in macrophages facilitates phagocytosis of apoptotic neutrophils. J Immunol. 2017;198:1660–72. https://doi.org/10.4049/jimmunol.1601495.
Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci U S A. 2000;97:680–5. https://doi.org/10.1073/pnas.97.2.680.
Al-Aqtash R, Ross MS, Collier DM. Extracellular histone proteins activate P2XR7 channel current. J Gen Physiol. 2023;155: e202213317. https://doi.org/10.1085/jgp.202213317.
Di Giovanni J, Iborra C, Maulet Y, Lévêque C, El Far O, Seagar M. Calcium-dependent regulation of SNARE-mediated membrane fusion by calmodulin. J Biol Chem. 2010;285:23665–75. https://doi.org/10.1074/jbc.M109.096073.
Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–7. https://doi.org/10.1126/science.1161748.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82170107, to ZMW), the Program of Shanghai Municipal Health Commission (202140406, to ZMW), the Excellent Subject Leader Program of Shanghai Municipal Health Commission (2022XD007, to ZMW), and the Foundation of the Science and Technology Commission of Shanghai Municipality (19ZR1443000 and 23ZR1453300, to ZMW).
Funding
National Natural Science Foundation of China,82170107, Program of Shanghai Municipal Health Commission, 202140406, Foundation of the Science and Technology Commission of Shanghai Municipality,19ZR1443000, 23ZR1453300, Excellent Subject Leader Program of Shanghai Municipal Health Commission, 2022XD007.
Author information
Authors and Affiliations
Contributions
ZMW secured the funding to support the project and the overall study. ZMW and CC conceptualized, designed, and supervised the study. JY, YF and NZ conducted the experiments, analyzed the results, and contributed to the manuscript writing. JMG, ZYZ and XMJ assisted in performing the experiments and provided technical support. ZMW and CC reviewed and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, J., Fu, Y., Zhang, N. et al. Extracellular histones promote TWIK2-dependent potassium efflux and associated NLRP3 activation in alveolar macrophages during sepsis-induced lung injury. Inflamm. Res. (2024). https://doi.org/10.1007/s00011-024-01888-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00011-024-01888-3