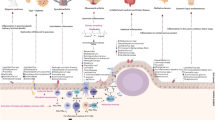
Abstract
Janus kinase/signal transduction and transcriptional activator (JAK/STAT) signaling pathway is a transport hub for cytokine secretion and exerts its effects. The activation of JAK/STAT signaling pathway is essential for the regulation of inflammatory responses. Inappropriate activation or deletion of JAK/STAT signaling pathway is the initiator of the inflammatory response. JAK/STAT signaling pathway has been demonstrated to be involved in the process of innate and adaptive immune response to inflammatory bowel disease (IBD). In this review, we discuss the role of the JAK/STAT signaling pathway in the regulation of different cells in IBD, as well as new findings on the involvement of the JAK/STAT signaling pathway in the regulation of the intestinal immune response. The current status of JAK inhibitors in the treatment of IBD is summarized as well. This review highlights natural remedies that can serve as potential JAK inhibitors. These phytochemicals may be useful in the identification of precursor compounds in the process of designing and developing novel JAK inhibitors.


Similar content being viewed by others
References
Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. https://doi.org/10.1038/s41572-020-0205-x.
Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, et al. Crohn’s disease. Nat Rev Dis Primers. 2020;6:22. https://doi.org/10.1038/s41572-020-0156-2.
Bernstein CN, Burchill C, Targownik LE, Singh H, Roos LL. Events within the first year of life, but not the neonatal period, affect risk for later development of inflammatory bowel diseases. Gastroenterology. 2019;156:2190–7. https://doi.org/10.1053/j.gastro.2019.02.004.
Hedin CRH, Vavricka SR, Stagg AJ, Schoepfer A, Raine T, Puig L, et al. The pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J Crohn’s Colitis. 2019;13:541–54. https://doi.org/10.1093/ecco-jcc/jjy191.
Aschenbrenner D, Quaranta M, Banerjee S, Ilott N, Jansen J, Steere B, et al. Deconvolution of monocyte responses in inflammatory bowel disease reveals an IL-1 cytokine network that regulates IL-23 in genetic and acquired IL-10 resistance. Gut. 2020. https://doi.org/10.1136/gutjnl-2020-321731.
Wang S, Zhang S, Huang S, Wu Z, Pang J, Wu Y, et al. Resistant maltodextrin alleviates dextran sulfate sodium-induced intestinal inflammatory injury by increasing butyric acid to inhibit proinflammatory cytokine levels. BioMed Res Int. 2020;2020:7694734. https://doi.org/10.1155/2020/7694734.
Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–78. https://doi.org/10.1038/nrgastro.2016.208.
O’Shea JJ. Targeting the Jak/STAT pathway for immunosuppression. Ann Rheum Dis. 2004;63(2):ii67–71. https://doi.org/10.1136/ard.2004.028290.
Wang A, Singh K, Ibrahim W, King B, Damsky W. The promise of JAK inhibitors for treatment of sarcoidosis and other inflammatory disorders with macrophage activation: a review of the literature. Yale J Biol Med. 2020;93:187–95.
Shivaji UN, Nardone OM, Cannatelli R, Smith SC, Ghosh S, Iacucci M. Small molecule oral targeted therapies in ulcerative colitis. Lancet Gastroenterol Hepatol. 2020;5:850–61. https://doi.org/10.1016/S2468-1253(19)30414-5.
Boland BS, Sandborn WJ, Chang JT. Update on Janus kinase antagonists in inflammatory bowel disease. Gastroenterol Clin N Am. 2014;43:603–17. https://doi.org/10.1016/j.gtc.2014.05.011.
Ma C, Battat R, Dulai PS, Parker CE, Sandborn WJ, Feagan BG, et al. Innovations in oral therapies for inflammatory bowel disease. Drugs. 2019;79:1321–35. https://doi.org/10.1007/s40265-019-01169-y.
Bazan JF. Shared architecture of hormone binding domains in type I and II interferon receptors. Cell. 1990;61:753–4. https://doi.org/10.1016/0092-8674(90)90182-e.
Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, et al. IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol (Baltimore, Md: 1950). 2004;172:3775–83. https://doi.org/10.4049/jimmunol.172.6.3775.
Liu R, Moriggl R, Zhang D, Li H, Karns R, Ruan HB, et al. Constitutive STAT5 activation regulates Paneth and Paneth-like cells to control clostridium difficile colitis. Life Sci Alliance. 2019;2:e201900296. https://doi.org/10.26508/lsa.201900296.
Günther C, Ruder B, Stolzer I, Dorner H, He GW, Chiriac MT, et al. Interferon lambda promotes paneth cell death via STAT1 signaling in mice and is increased in inflamed ileal tissues of patients with Crohn’s disease. Gastroenterol. 2019;157:1310–22. https://doi.org/10.1053/j.gastro.2019.07.031.
Li H, Feng C, Fan C, Yang Y, Yang X, Lu H, et al. Intervention of oncostatin M-driven mucosal inflammation by berberine exerts therapeutic property in chronic ulcerative colitis. Cell Death Dis. 2020;11:271. https://doi.org/10.1038/s41419-020-2470-8.
Li Y, Li W, Fu C, Song Y, Fu Q. Lonicerae japonicae flos and Lonicerae flos: a systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem Rev. 2019;49:74–9. https://doi.org/10.1007/s11101-019-09655-7.
Martin-Gallausiaux C, Larraufie P, Jarry A, Béguet-Crespel F, Marinelli L, Ledue F, et al. Butyrate produced by commensal bacteria down-regulates expression a dual mechanism in human intestinal epithelial cells. Front Immunol. 2018;9:2838. https://doi.org/10.3389/fimmu.2018.02838.
Dambacher J, Beigel F, Seiderer J, Haller D, Göke B, Auernhammer CJ, et al. Interleukin 31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is upregulated in inflammatory bowel disease. Gut. 2007;56:1257–65. https://doi.org/10.1136/gut.2006.118679.
Gilbert S, Nivarthi H, Mayhew CN, Lo Y-H, Noah TK, Vallance J, et al. Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Rep. 2015;4:209–25. https://doi.org/10.1016/j.stemcr.2014.12.004.
Takashima S, Martin ML, Jansen SA, Fu Y, Bos J, Chandra D, et al. T cell-derived interferon-γ programs stem cell death in immune-mediated intestinal damage. Sci Immunol. 2019;4:eaay8556. https://doi.org/10.1126/sciimmunol.aay8556.
Richmond CA, Rickner H, Shah MS, Ediger T, Deary L, Zhou F, et al. JAK/STAT-1 signaling is required for reserve intestinal stem cell activation during intestinal regeneration following acute inflammation. Stem Cell Rep. 2018;10:17–26. https://doi.org/10.1016/j.stemcr.2017.11.015.
Peplowski MA, Dicay M, Baggio CH, Wysokinski F, Renaux B, Hollenberg MD, et al. Interferon gamma decreases intestinal epithelial aquaporin 3 expression through downregulation of constitutive transcription. J Mol Med (Berlin, Germany). 2018;96:1081–93. https://doi.org/10.1007/s00109-018-1681-2.
Dicay MS, Hirota CL, Ronaghan NJ, Peplowski MA, Zaheer RS, Carati CA, et al. Interferon-γ suppresses intestinal epithelial aquaporin-1 expression via Janus kinase and STAT3 activation. PLoS ONE. 2015;10:e0118713. https://doi.org/10.1371/journal.pone.0118713.
Gilbert S, Zhang R, Denson L, Moriggl R, Steinbrecher K, Shroyer N, et al. Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol Med. 2012;4:109–24. https://doi.org/10.1002/emmm.201100192.
Eissa N, Hussein H, Mesgna R, Bonin S, Hendy GN, Metz-Boutigue MH, et al. Catestatin Regulates epithelial cell dynamics to improve intestinal inflammation. Vaccines. 2018;6:67. https://doi.org/10.3390/vaccines6040067.
Wang Y, Mumm JB, Herbst R, Kolbeck R, Wang Y. IL-22 increases permeability of intestinal epithelial tight junctions by enhancing Claudin-2 expression. J Immunol (Baltimore, Md: 1950). 2017;199:3316–25. https://doi.org/10.4049/jimmunol.1700152.
Krishnan M, McCole DF. T cell protein tyrosine phosphatase prevents STAT1 induction of claudin-2 expression in intestinal epithelial cells. Ann NY Acad Sci. 2017;1405:116–30. https://doi.org/10.1111/nyas.13439.
Zhou X, Li W, Wang S, Zhang P, Wang Q, Xiao J, et al. YAP Aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019;27:1176–89. https://doi.org/10.1016/j.celrep.2019.03.028.
Zhang Y, Li X, Luo Z, Ma L, Zhu S, Wang Z, et al. ECM1 is an essential factor for the determination of M1 macrophage polarization in IBD in response to LPS stimulation. Proc Natl Acad Sci USA. 2020;117:3083–92. https://doi.org/10.1073/pnas.1912774117.
Najjar I, Fagard R. STAT1 and pathogens, not a friendly relationship. Biochimie. 2010;92:425–44. https://doi.org/10.1016/j.biochi.2010.02.009.
Li HS, Watowich SS. Innate immune regulation by STAT-mediated transcriptional mechanisms. Immunol Rev. 2014;261:84–101. https://doi.org/10.1111/imr.12198.
Giri J, Das R, Nylen E, Chinnadurai R, Galipeau J. CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ Tissue macrophages to mitigate gut injury. Cell Rep. 2020;30:1923–34. https://doi.org/10.1016/j.celrep.2020.01.047.
Vier J, Groth M, Sochalska M, Kirschnek S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell Death Dis. 2016;7:e2103. https://doi.org/10.1038/cddis.2016.23.
Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–66. https://doi.org/10.1038/mi.2012.24.
Stabile H, Scarno G, Fionda C, Gismondi A, Santoni A, Gadina M, et al. JAK/STAT signaling in regulation of innate lymphoid cells: the gods before the guardians. Immunol Rev. 2018;286:148–59. https://doi.org/10.1111/imr.12705.
Powell N, Pantazi E, Pavlidis P, Tsakmaki A, Li K, Yang F, et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut. 2020;69:578–90. https://doi.org/10.1136/gutjnl-2019-318483.
Cao Q, Gao X, Lin Y, Yue C, Wang Y, Quan F, et al. Thymopentin ameliorates dextran sulfate sodium-induced colitis by triggering the production of IL-22 in both innate and adaptive lymphocytes. Theranostics. 2019;9:7490–505. https://doi.org/10.7150/thno.35015.
Castellanos JG, Woo V, Viladomiu M, Putzel G, Lima S, Diehl GE, et al. Microbiota-induced TNF-like ligand 1A drives group 3 innate lymphoid cell-mediated barrier protection and intestinal T cell activation during colitis. Immunity. 2018;49:1077–89. https://doi.org/10.1016/j.immuni.2018.10.014.
Li HS, Yang CY, Nallaparaju KC, Zhang H, Liu Y-J, Goldrath AW, et al. The signal transducers STAT5 and STAT3 control expression of Id2 and E2–2 during dendritic cell development. Blood. 2012;120:4363–73. https://doi.org/10.1182/blood-2012-07-441311.
Yang C, Zhang Y, Wang J, Li L, Wang L, Hu M, et al. A novel cyclic helix B peptide inhibits dendritic cell maturation during amelioration of acute kidney graft rejection through Jak-2/STAT3/SOCS1. Cell Death Dis. 2015;6:e1993. https://doi.org/10.1038/cddis.2015.338.
Sottile R, Federico G, Garofalo C, Tallerico R, Faniello MC, Quaresima B, et al. Iron and ferritin modulate MHC class I expression and NK cell recognition. Front Immunol. 2019;10:224. https://doi.org/10.3389/fimmu.2019.00224.
Simpson JAD, Al-Attar A, Watson NFS, Scholefield JH, Ilyas M, Durrant LG. Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut. 2010;59:926–33. https://doi.org/10.1136/gut.2009.194472.
Yu C-F, Peng W-M, Schlee M, Barchet W, Eis-Hübinger AM, Kolanus W, et al. SOCS1 and SOCS3 Target IRF7 degradation to suppress TLR7-mediated type I IFN production of human plasmacytoid dendritic cells. J Immunol (Baltimore Md: 1950). 2018;200:4024–35. https://doi.org/10.4049/jimmunol.1700510.
Zhu Z, Zhao Y, Huo H, Gao X, Zheng J, Li J, et al. HHX-5, a derivative of sesquiterpene from Chinese agarwood, suppresses innate and adaptive immunity via inhibiting STAT signaling pathways. Eur J Pharmacol. 2016;791:412–23. https://doi.org/10.1016/j.ejphar.2016.09.023.
Obraztsov IV, Shirokikh KE, Obraztsova OI, Shapina MV, Wang M-H, Khalif IL. Multiple cytokine profiling: a new model to predict response to tumor necrosis factor antagonists in ulcerative colitis patients. Inflamm Bowel Dis. 2019;25:524–31. https://doi.org/10.1093/ibd/izy358.
Zhao H-M, Xu R, Huang X-Y, Cheng S-M, Huang M-F, Yue H-Y, et al. Curcumin Suppressed Activation of Dendritic Cells via JAK/STAT/SOCS Signal in Mice with Experimental Colitis. Front Pharmacol. 2016;7:455. https://doi.org/10.3389/fphar.2016.00455.
Poholek CH, Raphael I, Wu D, Revu S, Rittenhouse N, Uche UU, et al. Noncanonical STAT3 activity sustains pathogenic Th17 proliferation and cytokine response to antigen. J Exp Med. 2020;217:e20191761. https://doi.org/10.1084/jem.20191761.
Cătană C-S, Berindan Neagoe I, Cozma V, Magdaş C, Tăbăran F, Dumitraşcu DL. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2015;21:5823–30. https://doi.org/10.3748/wjg.v21.i19.5823.
Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123:939–44. https://doi.org/10.1172/JCI57175.
Liu M, Li S, Li MO. TGF-β control of adaptive immune tolerance: a break from treg cells. BioEssays. 2018;40:e1800063. https://doi.org/10.1002/bies.201800063.
Li H-B, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, et al. mA mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–42. https://doi.org/10.1038/nature23450.
Bitar M, Boldt A, Freitag M-T, Gruhn B, Köhl U, Sack U. Evaluating STAT5 phosphorylation as a mean to assess T cell proliferation. Front Immunol. 2019;10:722. https://doi.org/10.3389/fimmu.2019.00722.
Goetz CA, Harmon IR, O’Neil JJ, Burchill MA, Farrar MA. STAT5 activation underlies IL7 receptor-dependent B cell development. J Immunol (Baltimore Md: 1950). 2004;172:4770–8. https://doi.org/10.4049/jimmunol.172.8.4770.
Sun H, Bi J, Lei Q, Wan X, Jiang T, Wu C, et al. Partial enteral nutrition increases intestinal sIgA levels in mice undergoing parenteral nutrition in a dose-dependent manner. Int J Surg. 2018;49:74–9. https://doi.org/10.1016/j.ijsu.2017.12.011.
Zeng B, Wang D, Wang H, Chen T, Luo J, Xi Q, et al. Dietary soy protein isolate attenuates intestinal immunoglobulin and mucin expression in young mice compared with casein. Nutrients. 2020;12:2739. https://doi.org/10.3390/nu12092739.
Sandborn WJ, Feagan BG, Loftus EV, Peyrin-Biroulet L, Van Assche G, D’Haens G, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with crohn’s disease. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.01.047.
Sandborn WJ, Ghosh S, Panes J, Schreiber S, D’Haens G, Tanida S, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterol. 2020;158:2139–49. https://doi.org/10.1053/j.gastro.2020.02.030.
Sandborn WJ, Nguyen DD, Beattie DT, Brassil P, Krey W, Woo J, et al. Development of gut-selective pan-janus kinase inhibitor TD-1473 for ulcerative colitis: a translational medicine programme. J Crohn’s Colitis. 2020;14:1202–13. https://doi.org/10.1093/ecco-jcc/jjaa049.
Wang L (2017) Study on the extraction and isolation of Polysaccharide from Portulaca oleracea and its anti ulcerative colitis mechanism. Vol. Master's. China: Shaanxi University of traditional Chinese Medicine
Ma C, Lee JK, Mitra AR, Teriaky A, Choudhary D, Nguyen TM, et al. Systematic review with meta-analysis: efficacy and safety of oral Janus kinase inhibitors for inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:5. https://doi.org/10.1111/apt.15297.
FDA. An investigational study of experimental medication bms-986165 in participants with moderate to severe Crohn's Disease 2021;https://clinicaltrials.gov/ct2/show/record/NCT03599622?term=BMS-986165&draw=2&rank=26
D’Amico F, Fiorino G, Furfaro F, Allocca M, Danese S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin Invest Drugs. 2018;27:595–9. https://doi.org/10.1080/13543784.2018.1492547.
Sands BE, Sandborn WJ, Feagan BG, Lichtenstein GR, Zhang H, Strauss R, et al. Peficitinib, an oral janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J Crohn’s Colitis. 2018;12:1158–69. https://doi.org/10.1093/ecco-jcc/jjy085.
Curtis JR, Regueiro M, Yun H, Su C, DiBonaventura M, Lawendy N, et al. Tofacitinib treatment safety in moderate to severe ulcerative colitis: comparison of observational population cohort data from the ibm marketscan® administrative claims database with tofacitinib trial data. Inflamm Bowel Dis. 2020. https://doi.org/10.1093/ibd/izaa289.
Fenster M, Alayo QA, Khatiwada A, Wang W, Dimopoulos C, Gutierrez A, et al. Real-World effectiveness and safety of tofacitinib in Crohn’s disease and IBD-U: a multicenter study from the TROPIC consortium. Clin Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.cgh.2020.10.025.
Shukla T, Sands BE. Novel non-biologic targets for inflammatory bowel disease. Curr Gastroenterol Rep. 2019;21:22. https://doi.org/10.1007/s11894-019-0689-2.
Li B, Jiang N, Gu S. Effects of wumei pill and its disassembled prescriptions on IL-6/JAK/STAT3 signaling pathway and pathological changes of colonic mucosa in rats with ulcerative colitis. Pharmacol Clin Chin Materia Medica. 2016;32:14–7. https://doi.org/10.13412/j.cnki.zyyl.2016.01.004.
Tao M, Wang X, Wang A, Gao N, Pan J, Liu X, et al. Effect of jiaweiwumel decoction on regulatory T cells and interleukin-10 in a rat model of ulcerative colitis. J Tradit Chin Med. 2015;35:312–5. https://doi.org/10.1016/s0254-6272(15)30103-5.
Zhai Y-h, Zhu X-d, Yang Y, Li T, Qi Q-f. Effects of Tongxie Yaofang on the STAT3 mRNA and protein expressions of colonic mucosa in rats with experimental ulcerative colitis. Chin J Tradit Chin Med Pharmacol. 2017;32:2710–3.
Ji J, Chen Y. Effect of huangqin decoction on IL-6, JAK-STAT3 signal pathway and HMGB-1 expression in rats with ulcerative colitis. China J Chin Med. 2018;33:1297–301. https://doi.org/10.16368/j.issn.1674-8999.2018.07.307.
Lin J (2012) Effect of changyuning granule on JAK/STAT pathway in rats with ulcerative colitis. Vol. Master's. China: Heilongjiang University of traditional Chinese Medicine
Wang W, Li G, Su P, Xu Q, Zheng C, Li M, et al. Therapeutic efficacy of Jiawei Chaishao Liujun Granules for rats with ulcerative colitis and its effect on myeloid differentiation factor 88 expression in colonic tissues (in Chinese). Guangxi Med J. 2020;42:821–6. https://doi.org/10.11675/j.issn.0253-4304.2020.07.09.
He L (2016) Effect of Wenshen Jianpi method on the expression of SOCS2/3 gene and protein in colonic tissue of ulcerative colitis rats. Vol. Master's. China: Gansu University of traditional Chinese Medicine
Zhang X, Wu J, Ye B, Wang Q, Xie X, Shen H. Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complement Altern Med. 2016;16:299. https://doi.org/10.1186/s12906-016-1273-z.
Ashrafizadeh M, Rafiei H, Mohammadinejad R, Afshar EG, Farkhondeh T, Samarghandian S. Potential therapeutic effects of curcumin mediated by JAK/STAT signaling pathway: a review. Phytother Res. 2020;34:1745–60. https://doi.org/10.1002/ptr.6642.
Kim T-W, Shin J-S, Chung K-S, Lee Y-G, Baek N-I, Lee K-T. Anti-inflammatory mechanisms of Koreanaside A, a lignan isolated from the flower of, against LPS-induced macrophage activation and DSS-induced colitis mice: the crucial role of AP-1, NF-κB, and JAK/STAT signaling. Cells. 2019;8:1164. https://doi.org/10.3390/cells8101163.
Yang W, Zhou G, Yu T, Chen L, Yu L, Guo Y, et al. Critical role of ROCK2 activity in facilitating mucosal CD4 T cell activation in inflammatory bowel disease. J Autoimmun. 2018;89:125–38. https://doi.org/10.1016/j.jaut.2017.12.009.
Radwan RR, Karam HM. Resveratrol attenuates intestinal injury in irradiated rats via PI3K/Akt/mTOR signaling pathway. Environ Toxicol. 2020;35:223–30. https://doi.org/10.1002/tox.22859.
Serra D, Rufino AT, Mendes AF, Almeida LM, Dinis TCP. Resveratrol modulates cytokine-induced Jak/STAT activation more efficiently than 5-aminosalicylic acid: an in vitro approach. PLoS ONE. 2014;9:e109048. https://doi.org/10.1371/journal.pone.0109048.
Shi Z, Liu Z, Liu C, Wu M, Su H, Ma X, et al. Spectrum-effect relationships between chemical fingerprints and antibacterial effects of Lonicerae Japonicae Flos and Lonicerae Flos Base on UPLC and microcalorimetry. Front Pharmacol. 2016;7:12. https://doi.org/10.3389/fphar.2016.00012.
Nunes C, Almeida L, Barbosa RM, Laranjinha J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017;8:387–96. https://doi.org/10.1039/c6fo01529h.
Liang J, Lin Y, Lu Z, Xiong W, Chen D, Chen J, et al. Mechanism of Mangiferin on ulcerative colitis in mice based on network pharmacology. Chinese Tradit Patent Med. 2019;41:2503–8. https://doi.org/10.3969/j.issn.1001-1528.2019.10.046.
Liu Y, Liu X, Hua W, Wei Q, Fang X, Zhao Z, et al. Berberine inhibits macrophage M1 polarization via AKT1/SOCS1/NF-κB signaling pathway to protect against DSS-induced colitis. Int Immunopharmacol. 2018;57:121–31. https://doi.org/10.1016/j.intimp.2018.01.049.
Jin X, Wang J, Xia Z-M, Shang C-H, Chao Q-L, Liu Y-R, et al. Anti-inflammatory and anti-oxidative activities of paeonol and its metabolites through blocking MAPK/ERK/p38 signaling pathway. Inflammation. 2016;39:434–46. https://doi.org/10.1007/s10753-015-0265-3.
Liu S, Zhang S, Lv X, Lu J, Ren C, Zeng Z, et al. Limonin ameliorates ulcerative colitis by regulating STAT3/miR-214 signaling pathway. Int Immunopharmacol. 2019;75:105768. https://doi.org/10.1016/j.intimp.2019.105768.
Xu R, Yuan Y, Qi J, Zhou J, Guo X, Zhang J, et al. Elucidation of the intestinal absorption mechanism of loganin in the human intestinal Caco-2 cell model. Evid Based Complement Alternat Med. 2018;2018:8340563. https://doi.org/10.1155/2018/8340563.
Yuan J, Cheng W, Zhang G, Ma Q, Li X, Zhang B, et al. Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-кB pathway. Int Immunopharmacol. 2020;81:106240. https://doi.org/10.1016/j.intimp.2020.106240.
Liang T, Wang Z, Zhang Z. Therapeutic effect of allicin on TNBS induced colitis in rats and its IL-6/JAK/STAT3 pathway. Chin J Crit Care Med. 2017;37:321–3. https://doi.org/10.3969/j.issn.1002-1949.2017.z1.243.
Li C, Lun W, Zhao X, Lei S, Guo Y, Ma J, et al. Allicin alleviates inflammation of trinitrobenzenesulfonic acid-induced rats and suppresses P38 and JNK pathways in Caco-2 cells. Mediators Inflamm. 2015;2015:434692. https://doi.org/10.1155/2015/434692.
Pandurangan AK, Ismail S, Saadatdoust Z, Esa NM. Allicin alleviates dextran sodium sulfate- (DSS-) induced ulcerative colitis in BALB/c mice. Oxid Med Cell Longev. 2015;2015:605208. https://doi.org/10.1155/2015/605208.
Acknowledgements
We would like to acknowledge the contributions of colleagues, institutions, or agencies that aided the efforts of the authors.
Funding
This work was supported by the National Key Research and Development Project of China (2017YFD050160203), National Natural Science Foundation of China (81600440).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The literature search and data analysis were performed by LW, YH, BS. The draft of the manuscript was written by LW, YH, BS. YX, JW, DC critically revised the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Responsible Editor: H. Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Hu, Y., Song, B. et al. Targeting JAK/STAT signaling pathways in treatment of inflammatory bowel disease. Inflamm. Res. 70, 753–764 (2021). https://doi.org/10.1007/s00011-021-01482-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01482-x