Abstract
Objective
The aim of this study was to investigate the molecular mechanism of human bone marrow mesenchymal stem cells (hMSCs) secreting miR-26a exosomes on the function of skin fibroblasts.
Methods
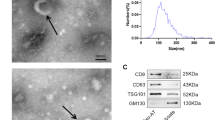
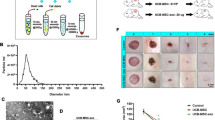
Exosomes from hMSCs were extracted and identified by transmission electron microscopy, particle size was analyzed and protein markers were detected. Then, the exosomes were co-cultured with human skin fibroblasts (BJ). CCK-8, Annexin V/P and Transwell assays were used to detect the proliferation, apoptosis, and migration of BJ cells. In addition, the expressions of miR-26a, related proteins, and related inflammatory factors were detected by qRT-PCR, western blotting, and ELISA.
Results
Compared with the high glucose group, the proliferation rate, migration rate, and the expression of α-SMA, bcl-2, TLR4, NF-κB, TNF-α, IL-6, IL- and IL-1 were significantly decreased in the high glucose + MSC-Exo-miR-26a mimics group, while the apoptosis rate and the expression of miR-26a, cleaved-caspase 3, cleaved-caspase 9 and Bax were significantly increased. The results of the high glucose + MSC-Exo-miR-26a inhibitor group were the opposite.
Conclusion
These results suggest that hMSCs cells secreting miR-26a exosomes inhibited the proliferation, migration, and transdifferentiation of high glucose-induced BJ cells, and promoted cell apoptosis, which may be related to the TLR4/NF-κB signaling pathway.








Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010;203(1–3):93–8.
Childs DR, Murthy AS. Overview of wound healing and management. Surg Clin North Am. 2017;97(1):189–207.
Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526.
Darby IA, Zakuan N, Billet F, Desmoulière A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci. 2015;73(6):1145–57.
Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology. Am J Pathol. 2012;180:1340–55.
Quaggin SE, Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 2011;80(1):41–50.
Suga H, Sugaya M, Fujita H, Asano Y, Tada Y, Kadono T, et al. TLR4, rather than TLR2, regulates wound healing through TGF-β and CCL5 expression. J Dermatol Sci. 2014;73(2):117–24.
Badiavas AR, Badiavas EV. Potential benefits of allogeneic bone marrow mesenchymal stem cells for wound healing. Expert Opin Biol Ther. 2011;11(11):1447–54.
Jiang L, Zhang Y, Zhu ZM, Gao F, Yang HH, Zhao ZG. Bone marrow mesenchymal stem cells accelerate wound healing in diabetic mice via inhibiting the expression of microRNA-155 to up-regulate Sirt1 in endothelial cells. Biomed Pharmacother. 2016;9(3):2980–8.
Qi Y, Dong Z, Chu H, Zhao Q, Jiang D. Denatured acellular dermal matrix seeded with bone marrow mesenchymal stem cells for wound healing in mice. Burns. 2019;45(7):1685–94.
Donker RB, Mouillet JF, Chu T, Hubel CA, Stolz DB, Morelli AE, Sadovsky Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod. 2012;18(8):417.
Zhong X, Zhang L, Li Y, Li P, Li J, Cheng G. Kaempferol alleviates ox-LDL-induced apoptosis by up-regulation of miR-26a-5p via inhibiting TLR4/NF-κB pathway in human endothelial cells. Biomed Pharmacother. 2018;108:1783–9.
Duan Y, Luo Q, Wang Y, Ma Y, Shi J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J Biol Chem. 2020;295(37):12868–84.
Zhang S, Cui R. The targeted regulation of miR-26a on PTEN-PI3K/AKT signaling pathway in myocardial fibrosis after myocardial infarction. Eur Rev Med Pharmacol Sci. 2018;22(2):523–31.
Song J. Glucose and amino acids affect mouse skin fibroblast growth through mTORC1 signaling pathway. Chongqing Med. 2013.
Ahn JY, Park S, Yun YS, Song JY. Inhibition of type III TGF-β receptor aggravates lung fibrotic process. Biomed Pharmacother. 2010;64(7):472–6.
Veksler-Lublinsky I, Shemer-Avni Y, Kedem K, Ziv-Ukelson M. Gene bi-targeting by viral and human miRNAs. BMC Bioinform. 2010;11(1):249.
Sorg RJH. Wound repair and regeneration. Eur Surg Res. 2012;25(1):79–92.
Eming SA, Krieg T, Davidson JM. Gene therapy and wound healing. Clin Dermatol. 2007;25(1):79–92.
Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23(11):1327–37.
Wang B, Zhang A, Wang H, Klein JD, Tan L, Wang ZM, Du J, Naqvi N, Liu BC, Wang XH. miR-26a limits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics. 2019;9(7):1864.
Qi J, Liu Y, Hu K, Zhang Y, Wu Y, Zhang X. MicroRNA-26a inhibits hyperplastic scar formation by targeting Smad2. Exp Ther Med. 2018;15(5):4332–8.
Jin M, Chadban SJ, Zhao CY, Xiaochen C, Tony K, Usha P, et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS ONE. 2014;9(5):e97985.
Jia P, Wu N, Jia D, Sun Y. Downregulation of MALAT1 alleviates saturated fatty acid-induced myocardial inflammatory injury via the miR-26a/HMGB1/TLR4/NF-κB axis. Diabetes Metab Syndr Obes Targets Ther. 2019;12:655–65.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81602742).
Author information
Authors and Affiliations
Contributions
QL and PH performed the research; PH designed the research study; WC contributed essential reagents or tools; WC and JB analyzed the data; QL wrote the paper; All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
There is no competing interest.
Ethics approval
The study protocol was approved by the Ethics Committee of Tongji Hospital.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Q., Huang, P., Chen, W. et al. Mechanism of bone marrow mesenchymal stem cells secreting miR-26a exosomes affecting high glucose-induced skin fibroblasts function by regulating TLR4/NF-κB signaling. Inflamm. Res. 70, 811–821 (2021). https://doi.org/10.1007/s00011-021-01478-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01478-7