Abstract
Background and aim
Intestinal epithelial dysfunction is the foundation of various intestinal and extra-intestinal diseases, while the effects and mechanism of uric acid on the intestinal barrier are little known. TSPO has been shown to be related to the generation of ROS and is involved in regulating inflammation, whether uric acid drives intestinal epithelial dysfunction through TSPO-mediated NLRP3 inflammasome activation is unknown.
Methods
UOX gene knockout mouse (UOX-/-) were used for models of hyperuricemia. Fluorescein isothiocyanate (FITC)-labeled dextran was used to assess in vivo intestinal permeability. Serum lipopolysaccharide (LPS) and culture supernatants IL-1β were measured using ELISA Kit. IEC-6 exposed to different concentrations of uric acid was used for in vitro experiment. Protein content and mRNA were assessed using Western blotting and Q-PCR, respectively. Intracellular ROS was determined using flow cytometry and fluorescence microscope. Mitochondrial membrane potential was detected on an immunofluorescence. Small interfering RNA transfection was used to assess the interaction between translocator protein (TSPO) and NLRP3 inflammasome. N-acetyl-L-cysteine (NAC) was used as ROS scavenger.
Results
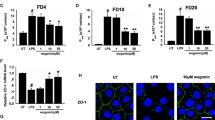
Our results showed that hyperuricemia mice were characteristic by increased intestinal permeability. Hyperuricemia upregulated TSPO, increased production of ROS and activated NLRP3 inflammasome, which resulted in lower expression of occludin and claudin-1. In vitro, we showed that soluble uric acid alone increased the expression of TSPO, depolarized mitochondrial membrane potential, increased ROS release and activated NLRP3 inflammasome, which further reduced the expression of occludin and claudin-1. Silencing TSPO suppressed NLRP3 inflammasome activation and increased expression of claudin-1 and occludin, which was accompanied by lower levels of ROS. Scavenging ROS also significantly inhibited NLRP3 inflammasome activation without change of TSPO, indicating that TSPO-mediated NLRP3 inflammasome activation was dependent on ROS.
Conclusions
In conclusion, uric acid drives intestinal barrier dysfunction through TSPO-mediated NLRP3 inflammasome.






Similar content being viewed by others
References
Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14(1):9–21. https://doi.org/10.1038/nrgastro.2016.169.
Chen M, Lu X, Lu C, Shen N, Jiang Y, Chen M, et al. Soluble uric acid increases PDZK1 and ABCG2 expression in human intestinal cell lines via the TLR4-NLRP3 inflammasome and PI3K/Akt signaling pathway. Arthritis Res Therapy. 2018;20(1):20. https://doi.org/10.1186/s13075-018-1512-4.
Hosomi A, Nakanishi T, Fujita T, Tamai I. Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PLoS ONE. 2012;7(2):e30456. https://doi.org/10.1371/journal.pone.0030456.
Braga TT, Forni MF, Correa-Costa M, Ramos RN, Barbuto JA, Branco P, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep. 2017;7:39884. https://doi.org/10.1038/srep39884.
Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–83. https://doi.org/10.1126/science.aar3318.
Munoz L, Borrero MJ, Ubeda M, Conde E, Del Campo R, Rodriguez-Serrano M, et al. Intestinal immune dysregulation driven by dysbiosis promotes barrier disruption and bacterial translocation in rats with cirrhosis. Hepatology (Baltimore, MD). 2019;70(3):925–38. https://doi.org/10.1002/hep.30349.
Monsuur AJ, Bakker PID, Alizadeh BZ, Zhernakova A, Bevova MR, Strengman E, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005;37(12):1341–4. https://doi.org/10.1038/ng1680.
Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7(1):31–40. https://doi.org/10.1038/nri1997.
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–85. https://doi.org/10.1038/nature10809.
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(9):688. https://doi.org/10.1038/nrd.2018.149.
Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–53. https://doi.org/10.1016/j.immuni.2013.05.016.
Daniels MJ, Rivers-Auty J, Schilling T. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer's disease in rodent models. Nat Commun. 2016;7:12504.
Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171(5):1110–24.e18. https://doi.org/10.1016/j.cell.2017.09.039.
Bordon Y. Trans-Golgi network breaks away to activate NLRP3. Nat Rev Immunol. 2019;19(2):68–9. https://doi.org/10.1038/s41577-018-0111-6.
Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21(3):193–201. https://doi.org/10.1016/j.molmed.2014.11.008.
Gatliff J, East D, Crosby J, Abeti R, Harvey R, Craigen W, et al. TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy. 2014;10(12):2279–96. https://doi.org/10.4161/15548627.2014.991665.
Wang GL, Yuan HM, Wang ZF, Zong BB, Ye Y, Zhang J, et al. Soluble uric acid activates NLRP3 inflammasome in myocardial cells through down-regulating UCP2. [Sichuan da xue xue bao Yi xue ban] Journal of Sichuan University Medical science edition. 2018;49(4):512–7.
Lee JW, Kim LE, Shim HJ, Kim EK, Hwang WC, Min DS, et al. A translocator protein 18 kDa ligand, Ro5-4864, inhibits ATP-induced NLRP3 inflammasome activation. Biochem Biophys Res Commun. 2016;474(3):587–93. https://doi.org/10.1016/j.bbrc.2016.04.080.
Kuwabara M, Borghi C, Cicero AFG, Hisatome I, Niwa K, Ohno M, et al. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: a five-year cohort study in Japan. Int J Cardiol. 2018;261:183–8. https://doi.org/10.1016/j.ijcard.2018.03.045.
Biornstad P, Laffel L, Lynch J, Ei Ghormli L, Weinstock RS, Tollefsen SE, et al. Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diabetes Care. 2019;42(6):1120–8. https://doi.org/10.2337/dc18-2147.
Onuora S. Gout: short telomeres in gout linked with flares and CVD. Nat Rev Rheumatol. 2017;13(6):324. https://doi.org/10.1038/nrrheum.2017.58.
Kuwabara M, Hisatome I, Niwa K, Hara S, Roncal-Jimenez CA, Bjornstad P, et al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese cohort study. Hypertension (Dallas, Tex: 1979). 2018;71(1):78–86. https://doi.org/10.1161/hypertensionaha.117.10370.
Crisan TO, Cleophas MC, Oosting M, Lemmers H, Toenhake-Diikstra H, Netea MG, et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis. 2016;75(4):755–62. https://doi.org/10.1136/annrheumdis-2014-206564.
Wan X, Xu C, Lin Y, Lu C, Li D, Sang J, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. 2016;64(4):925–32. https://doi.org/10.1016/j.jhep.2015.11.022.
Ko J, Kang HJ, Kim DA, Kim MJ, Ryu ES, Lee S, et al. Uric acid induced the phenotype transition of vascular endothelial cells induction of oxidative stress and glycocalyx shedding. FASEB J. 2019;33:13334–455. https://doi.org/10.1096/fj.201901148R.
Winer DA, Luck H, Tsai S, Winer S. the intestinal immune system in obesity and insulin resistance. Cell Metab. 2016;23(3):413–26. https://doi.org/10.1016/j.cmet.2016.01.003.
Song D, Cheng Y, Li X, Wang F, Lu Z, Xiao X, et al. Biogenic nanoselenium particles effectively attenuate oxidative stress-induced intestinal epithelial barrier injury by activating the Nrf2 antioxidant pathway. ACS Appl Mater Interfaces. 2017;9(17):14724–40. https://doi.org/10.1021/acsami.7b03377.
Selvaraj V, Stocco DM. The changing landscape in translocator protein (TSPO) function. Trends Endocrinol Metabol TEM. 2015;26(7):341–8. https://doi.org/10.1016/j.tem.2015.02.007.
Ostuni MA, Issop L, Peranzi G, Walker F, Fasseu M, Elbim C, et al. Overexpression of translocator protein in inflammatory bowel disease: potential diagnostic and treatment value. Inflamm Bowel Dis. 2010;16(9):1476–87. https://doi.org/10.1002/ibd.21250.
Thai PN, Daugherty DJ, Frederich BJ, Lu X, Deng W, Bers DM. Cardiac-specific conditional knockout of the 18-kDa mitochondrial translocator protein protects from pressure overload induced heart failure. Sci Rep. 2018;8(1):16213. https://doi.org/10.1038/s41598-018-34451-2.
Elinav E, Strowig T, Henao-Mejia J, Flavell R. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34(5):665–79. https://doi.org/10.1016/j.immuni.2011.05.007.
Elinav E, Henao-Meiia J, Flavell RA. Integrative inflammasome activity in the regulation of intestinal mucosal immune responses. Mucosal Immunol. 2013;6(1):4–13. https://doi.org/10.1038/mi.2012.115.
Scaini G, Barichello T. TSPO upregulation in bipolar disorder and concomitant downregulation of mitophagic proteins and NLRP3 inflammasome activation. Neuropsychopharmacology. 2018. https://doi.org/10.1038/s41386-018-0293-4.
Xiao J, Zhang XL, Fu C, Han R, Chen W, Lu Y, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1beta expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med. 2015;35(5):1347–54. https://doi.org/10.3892/ijmm.2015.2148.
Nadatani Y, Huo X, Zhang X, Yu C, Cheng E, Zhang Q, et al. NOD-Like receptor protein 3 inflammasome priming and activation in Barrett's epithelial cells. Cell Mol Gastroenterol Hepatol. 2016;2(4):439–53. https://doi.org/10.1016/j.jcmgh.2016.03.006.
Alfonso-Loeches S, Ureña-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci. 2014;8:216. https://doi.org/10.3389/fncel.2014.00216.
He Y, Franchi L, Núñez G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190(1):334–9. https://doi.org/10.4049/jimmunol.1202737.
Gross CJ, Mishra R, Schneider KS, Medard G, Wettmarshausen J, Dittlein DC, et al. K(+) efflux-independent nlrp3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45(4):761–73. https://doi.org/10.1016/j.immuni.2016.08.010.
Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8(1):202. https://doi.org/10.1038/s41467-017-00227-x.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. https://doi.org/10.1038/nature09663.
Gatliff J, East DA, Singh A, Alvarez MS, Frison M, Matic I, et al. A role for TSPO in mitochondrial Ca(2+) homeostasis and redox stress signaling. Cell Death Dis. 2017;8(6):e2896. https://doi.org/10.1038/cddis.2017.186.
Yang Y, Zhou Y, Cheng S, Sun JL, Yao H, Ma L. Effect of uric acid on mitochondrial function and oxidative stress in hepatocytes. Genet Mol Res GMR. 2016. https://doi.org/10.4238/gmr.15028644.
Mills EL, Kelly B, O'Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18(5):488–98. https://doi.org/10.1038/ni.3704.
Afonina IS, Zhong Z, Karin M, Beyaert R. Limiting inflammation-the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat Immunol. 2017;18(8):861–9.
Ma C, Yang X, Lv Q, Yan Z, Chen Z, Xu D, et al. Soluble uric acid induces inflammation via TLR4/NLRP3 pathway in intestinal epithelial cells. Iran J Basic Med Sci. 2020;23(6):744–50. https://doi.org/10.22038/ijbms.2020.44948.10482.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (81671625, 81100554, 81901575), Shandong Province Natural Science Fund Project (ZR2018PH010 and ZR2014HM015), Young and Middle-Aged Scientists Research Awards Fund of Shandong Province (BS2012YY003), the Scientific and Technical Development Project of Department of Health of Shandong Province (2011QZ007 and 2016WS0259), a Project of Shandong Province Higher Educational Science and Technology Program (J14LK11) and the Scientific and Technical Development Project of Qingdao (12-1-4-20-jc, 2012-1-3-2-(1)-nsh, 2013-13-008-YY, 2014-1-72 and 17-3-3-15-nsh ).
Author information
Authors and Affiliations
Contributions
SX and GY designed the study and revised the manuscript. QL and DX carried out the experiments and wrote the manuscript. YW, WY and XY contributed significantly to analyzing all the data. XL, JM, PZ conducted the mouse experiment. ZL and LM revised the manuscript. All authors approved the final version of the article.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, Q., Xu, D., Ma, J. et al. Uric acid drives intestinal barrier dysfunction through TSPO-mediated NLRP3 inflammasome activation. Inflamm. Res. 70, 127–137 (2021). https://doi.org/10.1007/s00011-020-01409-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01409-y