Abstract
Objectives
Cytokines participate in the progression of acute respiratory distress syndrome (ARDS), and uncontrolled inflammation is a central issue of acute lung injury (ALI). Interleukin (IL)-33 is a nuclear protein that has been reported to have a proinflammatory role in ARDS. Studies have shown that excessive autophagy may lead to the increased mortality of patients with ARDS, while several investigations indicated that IL-33 and autophagy interact with one another. The present study sought to clarify the relation between autophagy and IL-33’s proinflammatory role in ARDS.
Methods
We built a lipopolysaccharide (LPS)-induced lung injury mouse model. To study the relationship between IL-33 and autophagy, mice were pretreated with rapamycin (RAPA; a promoter of autophagy) and 3-methyladenine (3-MA; an inhibitor of autophagy) prior to LPS administration. The expression of IL-33 in serum and bronchoalveolar lavage fluid (BALF) was measured. Immunohistochemistry of IL-33 in lung tissue was examined. Th1,Th2 cytokines/chemokine levels in serum and BALF were tested. Further, the severity of lung injury was evaluated. And the nuclear factor-kappa B (NF-κB)'s nuclear translocation in lung tissue was detected.
Results
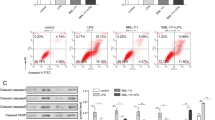
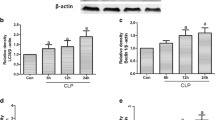
In comparison with the control group, the levels of IL-33 in serum and BALF were increased after LPS injection. Th1 cytokines/chemokine levels were significantly increased in serum and BALF, while Th2 cytokine levels changed only a little. The levels of IL-33 in serum and BALF of the RAPA group was significantly increased after LPS was injected as compared with the LPS group; additionally, the levels of IL-33 in serum and BALF of the 3-MA group was significantly reduced after LPS was injected as compared with the LPS group, and that lung injury was ameliorated after 3-MA pretreatment. Th1 cytokines and chemokines in both serum and BALF were also decreased in the 3-MA group. Furthermore, we found that the nuclear translocation of NF-κB increased after LPS administration, and NF-κB’s nuclear translocation was significantly increased in comparison with the LPS group after RAPA pretreatment. In contrast, NF-κB’s nuclear translocation decreased after 3-MA pretreatment as compared with the LPS group.
Conclusions
These findings showed that autophagy might regulate IL-33 by activating or inhibiting NF-κB to control the uncontrolled inflammation of acute lung injury.




Similar content being viewed by others
References
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800.
Chen W, Chen YY, Tsai CF, Chen SC, Lin MS, Ware LB, Chen CM. Incidence and outcomes of acute respiratory distress syndrome: a nationwide registry-based study in Taiwan, 1997 to 2011. Medicine (Baltimore). 2015;94(43):e1849.
Maca J, Jor O, Holub M, Sklienka P, Bursa F, Burda M, Janout V, Sevcik P. Past and present ARDS mortality rates: a systematic review. Respir Care. 2017;62(1):113–22.
Monahan LJ. Acute respiratory distress syndrome. Curr Probl Pediatr Adolesc Health Care. 2013;43(10):278–84.
Heinrich CH, Going SB, Pamenter RW, Perry CD, Boyden TW, Lohman TG. Bone mineral content of cyclically menstruating female resistance and endurance trained athletes. Med Sci Sports Exerc. 1990;22(5):558–63.
Burnum JF. Secrets about patients. N Engl J Med. 1991;324(16):1130–3.
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90.
Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–10.
Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3(10):e3331.
Lefrancais E, Cayrol C. Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members. Eur Cytokine Netw. 2012;23(4):120–7.
Braun H, Afonina IS, Mueller C, Beyaert R. Dichotomous function of IL-33 in health and disease: from biology to clinical implications. Biochem Pharmacol. 2018;148:238–52.
Zhang Y, Lv R, Hu X, Jiang L, Xiao D, Sun Y, Zhao J, Bao Q, Xie J. The role of IL-33 on LPS-induced acute lung injury in mice. Inflammation. 2017;40(1):285–94.
Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183(8):5094–103.
Le Goffic R, Arshad MI, Rauch M, L’Helgoualc’h A, Delmas B, Piquet-Pellorce C, Samson M. Infection with influenza virus induces IL-33 in murine lungs. Am J Respir Cell Mol Biol. 2011;45(6):1125–32.
Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard JP, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Investig. 2013;123(9):3967–82.
Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L, Graham GJ, Kurowska-Stolarska M, Liew FY, McSharry C, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 2014;134(6):1422–1432 e1411.
Martinez-Gonzalez I, Roca O, Masclans JR, Moreno R, Salcedo MT, Baekelandt V, Cruz MJ, Rello J, Aran JM. Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol. 2013;49(4):552–62.
Fu J, Lin SH, Wang CJ, Li SY, Feng XY, Liu Q, Xu F. HMGB1 regulates IL-33 expression in acute respiratory distress syndrome. Int Immunopharmacol. 2016;38:267–74.
Lin SH, Fu J, Wang CJ, Gao F, Feng XY, Liu Q, Cao J, Xu F. Inflammation elevated IL-33 originating from the lung mediates inflammation in acute lung injury. Clin Immunol. 2016;173:32–43.
Abebayehu D, Spence AJ, Qayum AA, Taruselli MT, McLeod JJ, Caslin HL, Chumanevich AP, Kolawole EM, Paranjape A, Baker B, et al. Lactic acid suppresses IL-33-mediated mast cell inflammatory responses via hypoxia-inducible factor-1alpha-dependent miR-155 suppression. J Immunol. 2016;197(7):2909–17.
Hu F, Shi L, Mu R, Zhu J, Li Y, Ma X, Li C, Jia R, Yang D, Li Y, et al. Hypoxia-inducible factor-1alpha and interleukin 33 form a regulatory circuit to perpetuate the inflammation in rheumatoid arthritis. PLoS One. 2013;8(8):e72650.
Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–8.
Position of beta blockers in antihypertensive therapy. J Cardiovasc Pharmacol. 1986;8(Suppl 6):S1–87.
Ma J, Sun Q, Mi R, Zhang H. Avian influenza A virus H5N1 causes autophagy-mediated cell death through suppression of mTOR signaling. J Genet Genom. 2011;38(11):533–7.
Wu J, Carlock C, Zhou C, Nakae S, Hicks J, Adams HP, Lou Y. IL-33 is required for disposal of unnecessary cells during ovarian atresia through regulation of autophagy and macrophage migration. J Immunol. 2015;194(5):2140–7.
Gao Y, Ma L, Luo CL, Wang T, Zhang MY, Shen X, Meng HH, Ji MM, Wang ZF, Chen XP, et al. IL-33 exerts neuroprotective effect in mice intracerebral hemorrhage model through suppressing inflammation/apoptotic/autophagic pathway. Mol Neurobiol. 2017;54(5):3879–92.
Campbell IK, Piccoli DS, Hamilton JA. Stimulation of human chondrocyte prostaglandin E2 production by recombinant human interleukin-1 and tumour necrosis factor. Biochim Biophys Acta. 1990;1051(3):310–8.
Blandino-Rosano M, Barbaresso R, Jimenez-Palomares M, Bozadjieva N, Werneck-de-Castro JP, Hatanaka M, Mirmira RG, Sonenberg N, Liu M, Ruegg MA, et al. Loss of mTORC1 signalling impairs beta-cell homeostasis and insulin processing. Nat Commun. 2017;8:16014.
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–23.
Morresi A, Simonacci M, Mozzicafreddo G, Giacchetti A, Criante P, Mambelli V, Sisti S, Marchesi E. Alclometasone dipropionate (Legederm) for the treatment of steroid-sensitive dermatoses in the elderly. G Ital Dermatol Venereol. 1990;125(6):XXIII–XXVII.
Pope DF, Cole KJ, Brand RA. Physiologic loading of the anterior cruciate ligament does not activate quadriceps or hamstrings in the anesthetized cat. Am J Sports Med. 1990;18(6):595–9.
Reiss LK, Schuppert A, Uhlig S. Inflammatory processes during acute respiratory distress syndrome: a complex system. Curr Opin Crit Care. 2018;24(1):1–9.
Hoste H. Trichostrongylus colubriformis: epithelial cell kinetics in the small intestine of infected rabbits. Exp Parasitol. 1989;68(1):99–104.
de la Vega MR, Dodson M, Gross C, Manzour H, Lantz RC, Chapman E, Wang T, Black SM, Garcia JG, Zhang DD. Role of Nrf2 and autophagy in acute lung injury. Curr Pharmacol Rep. 2016;2(2):91–101.
Casanova C, Freeman RD, Nordmann JP. Monocular and binocular response properties of cells in the striate-recipient zone of the cat’s lateral posterior-pulvinar complex. J Neurophysiol. 1989;62(2):544–57.
Liu Y, Zhang J. Saturated hydrogen saline ameliorates lipopolysaccharide-induced acute lung injury by reducing excessive autophagy. Exp Ther Med. 2017;13(6):2609–15.
Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci USA. 2010;107(44):18880–5.
Su Q, Eugster HP, Ryffel B, Dumont FJ. Cyclosporin A enhances the calcium-dependent induction of AP-1 complex and c-fos mRNA in a T cell lymphoma. Biochem Biophys Res Commun. 1996;229(1):249–56.
Chen XL, Li J, Xu GG, Li HX, Guo J. Rapamycin enhances IFN-gamma and IL-4 production in co-culture of gdelta T and dendritic cells from mice with lipopolysaccharide-induced acute lung injury. Genet Mol Res 2016;15(2):gmr7511(1–12).
Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36(12):2445–62.
Marks J. Benzodiazepines: a 1990 update. Proceedings of a symposium. January 5, 1990, New York City. Hosp Pract (Off Ed). 1990;25(Suppl 3):1–56.
Dominguez J, Mahalati K, Kiberd B, McAlister VC, MacDonald AS. Conversion to rapamycin immunosuppression in renal transplant recipients: report of an initial experience. Transplantation. 2000;70(8):1244–7.
Slavin SA, Leonard A, Grose V, Fazal F, Rahman A. Autophagy inhibitor 3-methyladenine protects against endothelial cell barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;314:L388–96.
Reiter P, Eliason DA, Francy DB, Moore CG, Campos EG. Apparent influence of the stage of blood meal digestion on the efficacy of ground applied ULV aerosols for the control of urban Culex mosquitoes. I. Field evidence. J Am Mosq Control Assoc. 1990;6(3):366–70.
Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50.
Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102.
Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–32.
Chu M, Chu IM, Yung EC, Lam CW, Leung TF, Wong GW, Wong CK. Aberrant expression of novel cytokine IL-38 and regulatory T lymphocytes in childhood asthma. Molecules. 2016;21(7):933(1–15).
Tao X, Song Z, Wang C, Luo H, Luo Q, Lin X, Zhang L, Yin Y, Cao J. Interleukin 36alpha attenuates sepsis by enhancing antibacterial functions of macrophages. J Infect Dis. 2017;215(2):321–32.
Zhao J, Wei J, Mialki RK, Mallampalli DF, Chen BB, Coon T, Zou C, Mallampalli RK, Zhao Y. F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nat Immunol. 2012;13(7):651–8.
Xu J, Guardado J, Hoffman R, Xu H, Namas R, Vodovotz Y, Xu L, Ramadan M, Brown J, Turnquist HR, et al. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: a reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14(7):e1002365.
Gao Y, Luo CL, Li LL, Ye GH, Gao C, Wang HC, Huang WW, Wang T, Wang ZF, Ni H, et al. IL-33 provides neuroprotection through suppressing apoptotic, autophagic and NF-kappaB-mediated inflammatory pathways in a rat model of recurrent neonatal seizure. Front Mol Neurosci. 2017;10:423.
Willart MA, Hammad H. Alarming dendritic cells for allergic sensitization. Allergol Int. 2010;59(2):95–103.
Lindley D. Old morality study finally surfaces. Nature. 1989;340(6229):90.
Weiskirchen R, Tacke F. Interleukin-33 in the pathogenesis of liver fibrosis: alarming ILC2 and hepatic stellate cells. Cell Mol Immunol. 2017;14(2):143–5.
Cayrol C, Girard JP. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev. 2018;281(1):154–68.
Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31(3):412–24.
Madalli S, Beyrau M, Whiteford J, Duchene J, Singh Nandhra I, Patel NS, Motwani MP, Gilroy DW, Thiemermann C, Nourshargh S, et al. Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol Sex Differ. 2015;6:27.
Traber KE, Hilliard KL, Allen E, Wasserman GA, Yamamoto K, Jones MR, Mizgerd JP, Quinton LJ. Induction of STAT3-dependent CXCL5 expression and neutrophil recruitment by oncostatin-M during pneumonia. Am J Respir Cell Mol Biol. 2015;53(4):479–88.
Moine P, Shenkar R, Kaneko D, Le Tulzo Y, Abraham E. Systemic blood loss affects NF-kappa B regulatory mechanisms in the lungs. Am J Physiol. 1997;273(1 Pt 1):L185–92.
Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, Abraham E. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med. 1996;24(8):1285–92.
Lentsch AB, Czermak BJ, Bless NM, Van Rooijen N, Ward PA. Essential role of alveolar macrophages in intrapulmonary activation of NF-kappaB. Am J Respir Cell Mol Biol. 1999;20(4):692–8.
Kessler M. Diagnosis of breast cancer. Krankenpfl J. 1989;27(12):22–9.
Shen X, Ma L, Dong W, Wu Q, Gao Y, Luo C, Zhang M, Chen X, Tao L. Autophagy regulates intracerebral hemorrhage induced neural damage via apoptosis and NF-kappaB pathway. Neurochem Int. 2016;96:100–12.
Acknowledgements
This study was supported by National Natural Science Foundation grants of China (81801894 to SH L, 81873928 to FX), Basic science and cutting-edge technology research projects of Chongqing Science and Technology Commission (cstc2016jcyjA0005, to FX), Special fund of social undertakings and people ‘s livelihood guarantee of Chongqing science and technology commission(cstc2017shmsA130072, to FX), the Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJ1702034, to FX),Medical research project of Chongqing City Health and Family Planning committee (2017ZDXM007 to FX, 2018MSXM097 to FY) and Chinese medicine science and technology project of Chongqing City Health and Family Planning committee (ZY201702071, to FX).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, M., Wang, Cj., Yu, F. et al. Different intensity of autophagy regulate interleukin-33 to control the uncontrolled inflammation of acute lung injury. Inflamm. Res. 68, 665–675 (2019). https://doi.org/10.1007/s00011-019-01250-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01250-y