Abstract
Objective and design
Abdominal aortic aneurysm (AAA) is heavily infiltrated with leukocytes, expressing the DNA sensor absent in melanoma 2 (AIM2) and other inflammasome components.
Methods
Using multicolour flow cytometry, we here compared the expression of the inflammasome components AIM2, NLRP3, and ASC in different peripheral immune cells derived from AAA patients with those from non-AAA patients in a case–control study. In parallel, peripheral blood mononuclear cells (PBMC) of AAA patients and controls were stimulated in vitro with poly-dA:dT or lipopolysaccharide (LPS) to analyze inflammasome activation.
Results
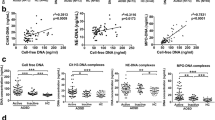
AIM2 expression was significantly increased in peripheral granulocytes (P = 0.026), monocytes (P = 0.007), B lymphocytes (P < 0.0001), and T lymphocytes (P = 0.004) of AAA patients. Expression of other inflammasome components did not differ between the groups. Following in vitro stimulation with foreign DNA, PBMC derived from AAA patients released significantly more IL-1β (P = 0.022) into the supernatant than PBMC from control patients. In contrast, IL-1β release upon LPS stimulation did not differ between the PBMC groups.
Conclusion
The data indicate the increased activation of an AIM2 inflammasome in peripheral immune cells of AAA patients and point to a systemic AIM2-associated immune response to AAA.



Similar content being viewed by others
References
Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE Jr, Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart. 2014;9:159–70.
Brewster DC, Cronenwett JL, Hallett JW Jr, Johnston KW, Krupski WC, Matsumura JS, Joint Council of the American Association for Vascular S, Society for Vascular S. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–17.
Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, van Herwaarden JA, Holt PJ, van Keulen JW, Rantner B, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):1–58.
Michel JB, Martin-Ventura JL, Egido J, Sakalihasan N, Treska V, Lindholt J, Allaire E, Thorsteinsdottir U, Cockerill G, Swedenborg J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27.
Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102.
Dale MA, Ruhlman MK, Baxter BT. Inflammatory cell phenotypes in AAAs: their role and potential as targets for therapy. Arterioscler Thromb Vasc Biol. 2015;35(8):1746–55.
Brophy CM, Reilly JM, Smith GJ, Tilson MD. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann Vasc Surg. 1991;5:229–33.
Newman KM, Jean-Claude J, Li H, Ramey WG, Tilson MD. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation. 1994;90:II224–227.
Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15:1145–51.
Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, Owens GK, Upchurch GR Jr, Ailawadi G. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33:294–304.
Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Yoshimura K, Aoki H, Tsutsui H, Noda T, et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:127–36.
Wu D, Ren P, Zheng Y, Zhang L, Xu G, Xie W, Lloyd EE, Zhang S, Zhang Q, Curci JA, et al. NLRP3 (nucleotide oligomerization domain-like receptor family, pyrin domain containing 3)-caspase-1 inflammasome degrades contractile proteins: implications for aortic biomechanical dysfunction and aneurysm and dissection formation. Arterioscler Thromb Vasc Biol. 2017;37:694–706.
Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411.
Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46:269–80.
Hakimi M, Peters A, Becker A, Bockler D, Dihlmann S. Inflammation-related induction of absent in melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions suggests a role in vascular pathogenesis. J Vasc Surg. 2014;59:794–803 (e792).
Dihlmann S, Erhart P, Mehrabi A, Nickkholgh A, Lasitschka F, Bockler D, Hakimi M. Increased expression and activation of absent in melanoma 2 inflammasome components in lymphocytic infiltrates of abdominal aortic aneurysms. Mol Med. 2014;20:230–7.
Wu X, Cakmak S, Wortmann M, Hakimi M, Zhang J, Bockler D, Dihlmann S. Sex- and disease-specific inflammasome signatures in circulating blood leukocytes of patients with abdominal aortic aneurysm. Mol Med. 2016; 22:508–518.
Wu X, Hakimi M, Wortmann M, Zhang J, Bockler D, Dihlmann S. Gene expression of inflammasome components in peripheral blood mononuclear cells (PBMC) of vascular patients increases with age. Immun Ageing. 2015;12:15.
Bengtsson H, Bergqvist D, Ekberg O, Janzon L. A population based screening of abdominal aortic aneurysms (AAA). Eur J Vasc Surg. 1991;5:53–7.
van Laarhoven CJ, Borstlap AC, van Berge Henegouwen DP, Palmen FM, Verpalen MC, Schoemaker MC. Chronic obstructive pulmonary disease and abdominal aortic aneurysms. Eur J Vasc Surg. 1993;7:386–90.
Bakele M, Joos M, Burdi S, Allgaier N, Poschel S, Fehrenbacher B, Schaller M, Marcos V, Kummerle-Deschner J, Rieber N, et al. Localization and functionality of the inflammasome in neutrophils. J Biol Chem. 2014;289:5320–9.
Svensson A, Patzi Churqui M, Schluter K, Lind L, Eriksson K. Maturation-dependent expression of AIM2 in human B-cells. PLoS One. 2017;12:e0183268.
Yang CA, Huang ST, Chiang BL. Sex-dependent differential activation of NLRP3 and AIM2 inflammasomes in SLE macrophages. Rheumatology. 2015;54:324–31.
Algaba-Chueca F, de-Madaria E, Lozano-Ruiz B, Martinez-Cardona C, Quesada-Vazquez N, Bachiller V, Tarin F, Such J, Frances R, Zapater P, Gonzalez-Navajas JM. The expression and activation of the AIM2 inflammasome correlates with inflammation and disease severity in patients with acute pancreatitis. Pancreatology. 2017;17:364–71.
Choubey D, Panchanathan R. Absent in melanoma 2 proteins in SLE. Clin Immunol. 2017;176:42–8.
Lupfer CR, Rodriguez A, Kanneganti TD. Inflammasome activation by nucleic acids and nucleosomes in sterile inflammation… or is it sterile? FEBS J. 2017;284:2363–74.
Lozano-Ruiz B, Bachiller V, Garcia-Martinez I, Zapater P, Gomez-Hurtado I, Moratalla A, Gimenez P, Bellot P, Frances R, Such J, Gonzalez-Navajas JM. Absent in melanoma 2 triggers a heightened inflammasome response in ascitic fluid macrophages of patients with cirrhosis. J Hepatol. 2015;62:64–71.
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91.
Harris J, Lang T, Thomas JPW, Sukkar MB, Nabar NR, Kehrl JH. Autophagy and inflammasomes. Mol Immunol. 2017;86:10–5.
Ocana E, Bohorquez JC, Perez-Requena J, Brieva JA, Rodriguez C. Characterisation of T and B lymphocytes infiltrating abdominal aortic aneurysms. Atherosclerosis. 2003;170:39–48.
Houard X, Touat Z, Ollivier V, Louedec L, Philippe M, Sebbag U, Meilhac O, Rossignol P, Michel JB. Mediators of neutrophil recruitment in human abdominal aortic aneurysms. Cardiovasc Res. 2009;82:532–41.
Acknowledgements
We thank Anja Spieler (Department of Vascular and Endovascular Surgery of the University Hospital Heidelberg, Germany) for excellent technical assistance.
Funding
We acknowledge financial support by the Department of Vascular and Endovascular surgery of the University Hospital Heidelberg, Germany. This research was supported in part by a grant of the Deutsche Forschungsgemeinschaft (DFG; No. 323488362) to MW and SD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Xianghui Xiao has received financial support from the German Society of Vascular Surgery and Vascular Medicine for attending the annual meeting of the society in 2017. Maani Hakimi is CEO of CODE Medical Frankfurt GbR, Germany. Dittmar Böckler is consultant for Medtronic, W.L Gore & Ass. Endologix. The authors have no competing interests. MW, GW, YS, and SD have no conflict of interest for this publication.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Responsible Editor: Andrew Roberts.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11_2019_1212_MOESM1_ESM.pdf
Gating strategy for phenotypic characterization of peripheral leukocytes that was applied for each individual AAA patient and age-matched subject without AAA. A. Live cells gated through the FSC-A versus SSC-A plot were selected and further characterized by plotting the SSC-A versus CD14-FITC, CD3-APC, or CD19-Cy5.5, respectively. Live CD14+ monocytes, CD3+ T cells and CD19+ B cells were then selected for analysis as shown in B. Granulocytes were defined as CD14- cells with a similar size a monocytes (SSC-A > 120k). B. Histograms of the cells gated as shown in A. The fluorescent intensities of PE (representing NLRP3, AIM2 or ASC, horizontal axis) are plotted against the number of events detected (vertical axis, normalized to mode) in different leukocyte phenotypes. Dotted lines refer to the background, as it was determined by analysis of cells incubated with a PE-labeled secondary antibody only. The solid line (grey peak) refers to the fluorescent intensities of the PE-labeled secondary antibody that was used in combination with anti-NLRP3, anti-AIM2 or anti-ASC specific antibodies. The example shown refers to a control sample. SSC-A: side scatter (reflecting granularity of detected cells), area scaling. (PDF 174 KB)
Rights and permissions
About this article
Cite this article
Wortmann, M., Xiao, X., Wabnitz, G. et al. AIM2 levels and DNA-triggered inflammasome response are increased in peripheral leukocytes of patients with abdominal aortic aneurysm. Inflamm. Res. 68, 337–345 (2019). https://doi.org/10.1007/s00011-019-01212-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01212-4