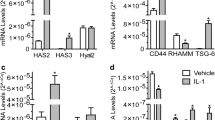
Abstract
Introduction
Our knowledge of extracellular matrix (ECM) structure and function has increased enormously over the last decade or so. There is evidence demonstrating that ECM provides signals affecting cell adhesion, shape, migration, proliferation, survival, and differentiation. ECM presents many domains that become active after proteolytic cleavage. These active ECM fragments are called matrikines which play different roles; in particular, they may act as potent inflammatory mediators during cartilage injury.
Findings
A major component of the ECM that undergoes dynamic regulation during cartilage damage and inflammation is the non-sulphated glycosaminoglycan (GAG) hyaluronan (HA). In this contest, HA is the most studied because of its different activity due to the different polymerization state. In vivo evidences have shown that low molecular weight HA exerts pro-inflammatory action, while high molecular weight HA possesses anti-inflammatory properties. Therefore, the beneficial HA effects on arthritis are not only limited to its viscosity and lubricant action on the joints, but it is especially due to a specific and effective anti-inflammatory activity. Several in vitro experimental investigations demonstrated that HA treatment may regulate different biochemical pathways involved during the cartilage damage. Emerging reports are suggesting that the ability to recognize receptors both for the HA degraded fragments, whether for the high-polymerized native HA involve interaction with integrins, toll-like receptors (TLRs), and the cluster determinant (CD44). The activation of these receptors induced by small HA fragments, via the nuclear factor kappa-light-chain enhancer of activated B cell (NF-kB) mediation, directly or other different pathways, produces the transcription of a large number of damaging intermediates that lead to cartilage erosion.
Conclusions
This review briefly summarizes a number of findings of the recent studies focused on the protective effects of HA, at the different polymerization states, on experimental arthritis in vitro both in animal and human cultured chondrocytes.





Similar content being viewed by others
References
Nogueira E, Gomes A, Preto A, Cavaco-Paulo A. Update on therapeutic approaches for rheumatoid arthritis. Curr Med Chem. 2016;23:2190–203.
Smolen JS, Aletaha D, Mclnnes IB. Rheumatoid arthritis. Lancet. 2016;22(388):2023–38.
Clegg TE, Caborn D, Mauffrey C. Viscosupplementation with hyaluronic acid in the treatment for cartilage lesions: a review of current evidence and future directions. Eur J Orthop Surg Traumatol. 2013;23:119–24.
Litwinium M, Krejner A, Speyrer MS, Gauto AR, Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28:78–88.
Fraser JRE, Laurent TC. Influence of serum on secretion of hyaluronic acid by synovial cells. Its possible relevance in arthritis. Ann Rheum Dis. 1969;28:419–23.
Henrotin Y, Lambert C, Richette P. Importance of synovitis in osteoarthritis: evidence for the use of glycosaminoglycans against synovial inflammation. Semin Arthritis Rheum. 2014;43:579–87.
Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human disease. Physiol Rev. 2011;91:221–64.
Gao Y, Liu S, Huang J, Guo W, Chen J, Zhang L, et al. The ECM-cell interaction of cartilage extracellular matrix on chon-drocytes. Biomed Res Int. 2014;2014:648459.
Valachova K, Volpi N, Stern R, Soltes L. Hyaluronan in medical practice. Curr Med Chem. 2016;23:3607–17.
Quero L, Klawitter M, Schmaus A, Rothley M, Sleeman J, Tiaden AN, et al. Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways. Arthitis Res Ther. 2013;15:R94. doi:10.1186/ar4274.
Cantor JO, Nadkarni PP. Hyaluronan the Jekyll and Hyde molecule. Inflamm Allergy Drug Targets. 2006;5:257–60.
Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015;. doi:10.1155/2015/563818.
Kavasi RM, Berdiaki A, Spyridaki I, Corsini E, Tsatsakis A, Tzanakakis G, et al. Ha metabolism in skin homeostasis and inflammatory disease. Food Chem Toxicol. 2017;101:128–38.
Cowman MK, Schmidt TA, Raghavan P, Stecco A. Viscoelastic properties of hyaluronan in physiological conditions. F1000Res. 2015;4:622. doi:10.12688/f1000research.6885.1.eCollection2015.
Hemshekhar M, Thushara RM, Chandranayaka S, Sherman LS, Kemparaju K, Girish KS. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int J Biol Macromol. 2016;86:917–28.
Adair-Kirk TL, Senior SM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–10.
Piombo V. Small animal models to understand pathogenesis of osteoarthritis and use of stem cell in cartilage regeneration. Cell Biochem Funct. 2017;35:3–11.
Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33.
Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drg Deliv Rev. 2016;97:186–203.
Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem. 2007;282:36777–81.
Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–92.
Rilla K, Oikari S, Jokela TA, Hyttinen JM, Karna R, Tammi RH, et al. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J Biol Chem. 2013;288:5973–83.
Triggs-Raine B, Natowicz MR. Biology of hyaluronan: insights from genetic disorders of hyaluronan metabolism. World J Biol Chem. 2015;6:110–20.
Fraser JR, Laurent TC, Engstrom-Laurent A, Laurent UG. Elimination of hyaluronic acid from the blood stream in the human. Clin Exp Pharmacol Physiol. 1984;11:17–25.
Stern R. Hyaluronidaes in cancer biology. Semin Cancer Biol. 2008;18:275–80.
Bastow ER, Byers S, Golub SB, Clarkin CE, Pitsillides AA, Fosang AJ. Hyaluronan synthesis and degradation in cartilage and bone. Cell Mol Life Sci. 2008;65:395–413.
McAtee CO, Barycki JJ, Simpson MA. Emerging roles for hyaluronidases in cancer metastasis and therapy. Adv Cancer Res. 2014;123:1–34.
Ragan C, Meyer K. The hyaluronic acid of synovial fluid in rheumatoid arthritis. J Clin Invest. 1949;28:56–9.
Manicourt DH, Triki R, Fukuda K, Devogelaer JP, Nagant de Deuxchaisnes C, Thonar EJ. Levels of circulating tumor necrosis factor alpha and interleukin-6 in patients with rheumatoid arthritis. Relationship to serum levels of hyaluronan and antigenic keratan sulfate. Arthritis Rheum. 1993;36:490–9.
Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1b-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26:1643–8.
Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Samà D, Calatroni A. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes. J Cell Biochem. 2009;106:83–92.
Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Calatroni A. Differential effect of molecular size HA in mouse chondrocytes stimulated with PMA. Biochim Biophys Acta. 2009;1790:1353–67.
Campo GM, Avenoso A, Campo S, Traina P, D’Ascola A, Calatroni A. Glycosaminoglycans reduced inflammatory response by modulating toll-like receptor-4 in LPS-stimulated chondrocytes. Arch Biochem Biophys. 2009;491:7–15.
Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie. 2010;92:2014–5.
Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Rugolo CA, Calatroni A. Differential effect of molecular mass hyaluronan on lipopolysaccharide-induced damage in chondrocytes. Innate Immun. 2010;16:48–63.
Miki Y, Teramura T, Tomiyama T, Onodera Y, Matsuoka T, Fukuda K, Hamanishi C. Hyaluronan reversed proteoglycan synthesis inhibited by mechanical stress: possible involvement of antioxidant effect. Inflamm Res. 2010;59:471–7.
Shaefer EC, Stewart AA, Durgam SS, Byron CR, Stewart MC. Effects of sodium hyaluronate and triamcinolone acetonide on glycosaminoglycans metabolism in equine articular chondrocytes treated with interleukin-1. Am J Vet Res. 2009;70:1494–501.
Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010;62:2004–12.
Ariyoshi W, Takahashi N, Hida D, Knudson CB, Knudson W. Mechanisms involved in enhancement of the expression and function of aggrecanases by hyaluronan oligosaccharides. Arthritis Rheum. 2012;64:187–97.
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Calatroni A, Campo S. Hyaluronan in part mediates IL-1beta-induced inflammation in mouse chondrocytes by up-regulating CD44 receptors. Gene. 2012;494:24–35.
Campo GM, Avenoso A, D’Ascola A, Prestipino V, Schuruchi M, Nastasi G, et al. Hyaluronan differently modulates TLR-4 and the inflammatory response in mouse chondrocytes. BioFactors. 2012;38:69–76.
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Nastasi G, et al. Adenosine A2A receptor activation and hyaluronan fragment inhibition reduce inflammation in mouse articular chondrocytes stimulated with interleukin-1b. FEBS J. 2012;279:2120–33.
Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, et al. The stimulation of adenosine 2° receptor reduces inflammatory response in mouse articular chondrocytes treated with hyaluronan oligosaccharides. Matrix Biol. 2012;31:338–51.
Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, et al. Protein Kinase a mediated anti-inflammatory effects exerted by adenosine treatment in mouse chondrocytes stimulated with IL-1β. BioFactors. 2012;38:429–39.
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Nastasi G, Micali A, et al. The SOD mimic MnTM-2-PyP(5 +) reduces hyaluronan degradation-induced inflammation in mouse articular chondrocytes stimulated with Fe(II) plus ascorbate. Int J Biochem Cell Biol. 2013;45:1610–9.
Chang CH, Hsu YM, Chen YC, Lin FH, Sadhasivam S, Loo ST, Savitha S. Anti-inflammatory effects of hydrophilic and lipophilic statins with hyaluronic acid against LPS-induced inflammation in porcine articular chondrocytes. J Orthop Res. 2014;32:557–65.
Mladenovic Z, Saurel AS, Berenbaum F, Jacques C. Potential role of hyaluronic acid on bone in osteoarthritis: matrix metalloproteinases, aggrecanases, and RANKL expression are partially prevented by hyaluronic acid in interleukin-1-stimulated osteoblasts. J Rheumatol. 2014;41:945–54.
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Calatroni A, Campo S. Beta-arrestin-2 negatively modulates inflammation response in mouse chondrocytes induced by 4-mer hyaluronan oligosaccharide. Mol Cell Biochem. 2015;399:201–8.
Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Calatroni A, Campo S. Beta-arrestin 1 is involved in the catabolic response stimulated by hyaluronan degradation in mouse chondrocytes. Cell Tissue Res. 2015;361:567–79.
Euppayo T, Siengdee P, Buddhachat K, Pradit W, Viriyakhasem N, Chomdej S, et al. Effects of low molecular weight hyaluronan combined with carprofen on canine osteoarthritis articular chondrocytes and cartilage explants in vitro. In Vitro Cell Dev Biol Anim. 2015;51:857–65.
Onodera Y, Teramura T, Takehara T, Fukuda K. Hyaluronic acid regulates a key redox control factor Nrf2 via phosphorylation of Akt in bovine articular chondrocytes. FEBS Open Bio. 2015;5:476–84.
Siengdee P, Euppayo T, Buddhachat K, Chomdej S, Nganvongpanit K. Two fluoroquinolones and their combinations with hyaluronan: comparison of effects on canine chondrocyte culture. J Vet Pharmacol Ther. 2016;39:439–51.
Euppayo T, Siengdee P, Buddhachat K, Pradit W, Chomdej S, Ongchai S, Nganvongpanit K. In vitro effects of triamcinolone acetonide and in combination with hyaluronan on canine normal and spontaneous osteoarthritis articular cartilage. In Vitro Cell Dev Biol Anim. 2016;52:723–35.
Ichimaru S, Nakagawa S, Arai Y, Kishida T, Shin-Ya M, Honjo K, et al. Hypoxia potentiates anabolic effect of exogenous hyaluronic acid in rat articular cartilage. Int J Mol Sci. 2016;17:E1013.
Qiu B, Gong M, He QT, Zhou PH. Controlled release of interleukin-1 receptor antagonist from hyaluronic acid-chitosan microspheres attenuates interleukin-1b-induced inflammation and apoptosis in chondrocytes. Biomed Res Int. 2016;2016:6290957.
Haschizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthr Cartil. 2009;17:1513–8.
Surazynski A, Miltyk W, Czarnomysy R, Grabowska J, Palka J. Hyaluronic acid abrogates nitric oxide-dependent stimulation of collagen degradation in cultured human chondrocytes. Pharmacol Res. 2009;60:46–9.
Peng H, Zhou JL, Liu SQ, Hu QJ, Ming JH, Qiu B. Hyaluronic acid inhibits nitric oxide –induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res. 2010;59:519–30.
Yasuda T. Comparison of hyaluronan effects among normal, osteoarthritis and rheumatoid arthritis cartilage stimulated with fibronectin fragments. Biomed Res. 2010;31:63–9.
Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharmacol. 2010;80:480–90.
Yasuda T. Hyaluronan inhibits p38 mitogen-activated protein kinase via the receptors in rheumatoid arthritis chondrocytes stimulated with fibronectin fragment. Clin Rheumatol. 2010;29:1259–67.
Hashizume M, Mihara M. High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1. Biochem Biophys Res Commun. 2010;403:184–9.
Julovi SM, Ito H, Nishitani K, Jackson CJ, Nakamura T. Hyaluronan inhibits matrix metalloproteinase-13 in human arthritic chondrocytes via CD44 and p38. J Orthop Res. 2011;29:258–64.
Hiraiwa H, Sakai T, Mitsuyama H, Hamada T, Yamamoto R, Omachi T, et al. Inflammatory effect of advanced glycation end products on human meniscal cells from osteoarthritic knees. Inflamm Res. 2011;60:1039–48.
Yasuda T. Activation of p38 mitogen-activated protein kinase is inhibited by hyaluronan via intercellular adhesion molecule-1 in articular chondrocytes stimulated with type II collagen peptide. J Pharmacol Sci. 2012;118:25–32.
Brun P, Zavan B, Vindigni V, Chiavinato A, Pozzuoli A, Iacobellis C, Abatangelo G. In vitro response of osteoarthritic chondrocytes and fibroblast-like synoviocytes to a 500–730 kDa hyaluronan amide derivative. J Biomed Mater Res B Appl Biomater. 2012;100:2073–81.
Murata M, Yudoh K, Shimizu H, Beppu M, Nakamura H, Kato T, Masuko K. Layilin, a talin-binding hyaluronan receptor, is expressed in human articular chondrocytes and synoviocytes and is down-regulated by interleukin-1β. Mod Rheumatol. 2013;23:478–88.
Prasadam I, Mao X, Shi W, Crawford R, Xiao Y. Combination of MEK-ERK inhibitor and hyaluronic acid has a synergistic effect on anti-hypertrophic and pro-chondrogenic activities in osteoarthritis treatment. J Mol Med. 2013;91:369–80.
Yasuda T. Nuclear factor-kB activation by type II collagen peptide in articular chondrocytes: its inhibition by hyaluronan via the receptors. Mod Rheumatol. 2013;23:1116–23.
Yu CJ, Ko CJ, Hsieh CH, Chien CT, Huang LH, Lee CW, Jiang CC. Proteomic analysis of osteoarthritic chondrocyte reveals the hyaluronic acid-regulated proteins involved in chondroprotective effect under oxidative stress. J Proteom. 2014;99:40–53.
Yoshioka K, Yasuda Y, Kisukeda T, Nodera R, Tanaka Y, Miyamoto K. Pharmacological effects of novel cross-linked hyaluronate, Gel-200, in experimental animal models of osteoarthritis and human cell lines. Osteoarthr Cartil. 2014;22:879–87.
Mongkon JM, Thach M, Shi Q, Fernandes JC, Fahmi H, Benderdour M. Sorbitol-modified hyaluronic acid reduces oxidative stress, apoptosis and mediators of inflammation and catabolism in human osteoarthritic chondrocytes. Inflamm Res. 2014;63:691–701.
Yasuda T. Type II collagen peptide stimulates Akt leading to nuclear-kB activation: its inhibition by hyaluronan. Biomed Res. 2014;35:193–9.
Chen WH, Lo WC, Hsu WC, Wei HJ, Liu HY, Lee CH, et al. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014;35:9599–607.
Campo GM, Micali A, Avenoso A, D’Ascola A, Scuruchi M, Pisani A, et al. Inhibition of small HA fragment activity and stimulation of A2A adenosine receptor pathway limit apoptosis and reduce cartilage damage in experimental arthritis. Histochem Cell Biol. 2015;143:531–43.
Ozawa M, Nishida K, Yoshida A, Saito T, Harada R, Machida T, Ozaki T. Hyaluronan suppresses mechanical stress-induced expression of catabolic enzymes by human chondrocytes via inhibition of IL-1β production and subsequent NF-κB activation. Inflamm Res. 2015;64:243–52.
Lee YJ, Kim SA, Lee SH. Hyaluronan suppresses lidocaine-induced apoptosis of human chondrocytes in vitro by inhibiting the p53-dependent mitochondrial apoptotic pathway. Acta Pharmacol Sin. 2016;37:664–73.
Furuta J, Ariyoshi W, Okinaga T, Takeuchi J, Mitsugi S, Tominaga K, Nishihara T. High molecular weight hyaluronic acid regulates MMP-13 expression in chondrocytes via DUSP10/MKP5. J Orthop Res. 2017;35:331–9.
Chen WH, Lin CM, Huang CF, Hsu WC, Lee CH, Ou KL, et al. Functional recovery in osteoarthritic chondrocytes through hyaluronic acid and platelet-rich plasma-inhibited infrapatellar fat pad adipocytes. Am J Sports Med. 2016;44:2696–705.
Bauer C, Niculescu-Morzsa E, Jeyakumar V, Kern D, Spath SS, Nehrer S. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. J Inflamm (Lond). 2016;13:31.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Avenoso, A., D’Ascola, A., Scuruchi, M. et al. Hyaluronan in the experimental injury of the cartilage: biochemical action and protective effects. Inflamm. Res. 67, 5–20 (2018). https://doi.org/10.1007/s00011-017-1084-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1084-9