Abstract
Background and aim
Liver inflammation stimulates various inflammatory cytokines and initiates injury through oxidative stress. The aim of this study was to curtaile the liver injury through natural principles such as 2-hydroxy-4-methoxy benzoic acid (HMBA).
Methods
The current study examines the hepatoprotective and lipid lowering effect of HMBA against carbon tetra chloride (CCl4)-mediated liver toxicity in male Wistar rats.
Results
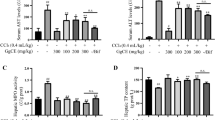
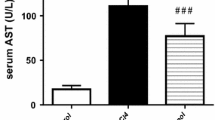
The hepatoprotective effects of HMBA against CCl4-induced liver damage, were evident from low serum transaminases activities, reduced hepatic lipid peroxidation and collagen content, restoration of total glutathione, and recouping of the inflammatory cytokines, such as TNF-α, IL-1β, IL-10, and IL-6 levels. Further it was found that the treatment of HMBA, significantly lowered (P<0.01) the levels of total cholesterol, triglycerides, free fatty acids and phospholipids in serum and liver. To investigate the mechanism behind the hepatoprotective and lipid lowering effect, the activities of heme oxygenase (HO1), and myeloperoxidase (MPO) were measured and expression levels were quantified through western blot following HMBA administration. The results showed that HMBA administration significantly decreased the activity of HO1 (P<0.001), and increased the activity of MPO (P<0.001); further similar finding was observed in western analysis. The hepatoprotective, lipid lowering and shifting key defensive enzyme activities are similar to that of standard drug such as N-acetylcysteine.
Conclusion
HMBA is competent of shielding liver from CCl4-induced hepatotoxicity, and this is associated with the lipid lowering, inflammatory cytokine restoration and induction of defensive enzyme activities.





Similar content being viewed by others
References
Michalopoulos GK. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384–92.
Wang S, Shi XL, Feng M, Wang X, Zhang ZH, Zhao X, et al. Puerarin protects against CCl4-induced liver fibrosis in mice: possible role of PARP- 1 inhibition. Int Immunopharmacol. 2016;38:238–45.
Li R, Wang Y, Zhao E, Wu K, Li W, Shi L, et al. Maresin, a proresolving lipid mediator, mitigates carbon tetrachloride-induced liver injury in mice. Oxid Med Cell Longev. 2016;2016:9203716.
Francisqueti FV, Chiaverini LC, Santos KC, Minatel IO, Ronchi CB, Ferron AJ, et al. The role of oxidative stress on the pathophysiology of metabolic syndrome. Rev Assoc Med Bras. 1992;63:85–91.
Sorokin A. Nitric oxide synthase and cyclooxygenase pathways: a complex interplay in cellular signaling. Curr Med Chem. 2016;23:2559–78.
Arcucci A, Ruocco MR, Granato G, Sacco AM, Montagnani S. Cancer: an oxidative crosstalk between solid tumor cells and cancer associated fibroblasts. Biomed Res Int. 2016;2016:4502846.
Forman HJ. Redox signaling: an evolution from free radicals to aging. Free Radic Biol Med. 2016;97:398–407.
Tibaut M, Petrovič D. Oxidative stress genes, antioxidants and coronary artery disease in type 2 diabetes mellitus. Cardiovasc Hematol Agents Med Chem. 2016;14:23–38.
Lushchak VI. Free radicals, reactive oxygen species, oxidative stresses and their classifications. Ukr Biochem J. 2015;87:11–8.
Gasparovic AC, Milkovic L, Sunjic SB, Zarkovic N. Cancer growth regulation by 4-hydroxynonenal. Free Radic Biol Med. 2017. doi:10.1016/j.freeradbiomed.2017.01.030
Hawk MA, McCallister C, Schafer ZT. Antioxidant activity during tumor progression: a necessity for the survival of cancer cells? Cancers (Basel). 2016;8:113–21.
Crosas-Molist E, Bertran E, Fabregat I. Cross-talk between TGF-β and NADPH oxidases during liver fibrosis and hepatocarcinogenesis. Curr Pharm Des. 2015;21:5964–76.
Tsukiyama-Kohara K. Role of oxidative stress in hepatocarcinogenesis induced by hepatitis C virus. Int J Mol Sci. 2012;13:15271–8.
Xie Y, Hao H, Wang H, Guo C, Kang A, Wang G. Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress. J Ethnopharmacol. 2014;155:213–21.
Taniguchi M, Takeuchi T, Nakatsuka R, Watanabe T, Sato K. Molecular process in acute liver injury and regeneration induced by carbon tetrachloride. Life Sci. 2004;75:1539–49.
Cui Y, Yang X, Lu X, Chen J, Zhao Y. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl4-induced liver injury in mice. Chem Biol Interact. 2014;220:75–83.
Rivera H, Shibayama M, Tsutsumi V, Perez-Alvarez V, Muriel P. Resveratrol and trimethylated resveratrol protect from acute liver damage induced by CCl4 in the rat. J Appl Toxicol. 2008;28:147–55.
Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–90.
Das S, Bisht SS. The bioactive and therapeutic potential of Hemidesmus indicus R. Br. (Indian Sarsaparilla) root. Phytother Res. 2013;27:791–801.
Sethi A, Bhatia A, Srivastava S, Bhatia G, Khan MM, Khanna AK, et al. Pregnane glycoside from Hemidesmus indicus as a potential anti-oxidant and anti-dyslipidemic agent. Nat Prod Res. 2010;24:1371–8.
Alam MI, Gomes A. Viper venom-induced inflammation and inhibition of free radical formation by pure compound (2-hydroxy-4-methoxy benzoic acid) isolated and purified from anantamul (Hemidesmus indicus R. BR) root extract. Toxicon. 1998;36:207–15.
Saravanan N, Nalini N. Effect of 2-hydroxy 4-methoxy benzoic acid on an experimental model of hyperlipidaemia, induced by chronic ethanol treatment. J Pharm Pharmacol. 2007;59:1537–42.
Saravanan N, Nalini N. Inhibitory effect of Hemidesmus indicus and its active principle 2-hydroxy 4-methoxy benzoic acid on ethanol-induced liver injury. Fundam Clin Pharmacol. 2007;21:507–14.
Gayathri M, Kannabiran K. Effect of 2-hydroxy-4-methoxy benzoic acid from the roots of Hemidesmus indicus on streptozotocin-induced diabetic rats. Indian J Pharm Sci. 2009;71:581–5.
Gayathri M, Kannabiran K. Effect of 2-hydroxy-4-methoxy benzoic acid isolated from Hemidesmus indicus on erythrocyte membrane bound enzymes and antioxidant status in streptozotocin-induced diabetic rats. Indian J Pharm Sci. 2012;74:474–8.
Gandhi CR, Sproat LA, Subbotin VM. Increased hepatic endothelin-1 levels and endothelin receptor density in cirrhotic rats. Life Sci. 1996;58:55–62.
Lowry OH, Rosebrough NJ, Farr AL, Randall RL. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212.
Bradbury P, Gordon KC. Connective tissue and stain. In: Bancroft JD, Stevens A, editors. Theory and practice of histological techniques. New York: Churchill Livingston Press; 1990. p. 119–42.
Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–22.
Maines M. Carbon monoxide and nitric oxide homology: differential modulation of heme oxygenases in brain and detection of protein and activity. Methods Enzymol. 1996;268:473–88.
Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1951;226:497–509.
Parekh AL, Jung DH. Cholesterol determination with ferric chloride-uranyl acetate and sulphuric acid ferrous sulphate reagent. Anal Biochem. 1970;42:1423–7.
Rouser G, Fleisher S, Yamanoto A. Two-dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–6.
Rice EW. Triglycerides (neutral fats) in serum. In: Mac-Donald RP, editor. Standard methods of clinical chemistry, vol. 6. New York: Academic Press; 1970. p. 215–222.
Hron WT, Menahan LA. A sensitive method for the determination of free fatty acids in plasma. J Lipid Res. 1981;23:377–81.
Saravanan N, Rajasankar S, Nalini N. Antioxidant effect of 2-hydroxy-4-methoxy benzoic acid on ethanol-induced hepatotoxicity in rats. J Pharm Pharmacol. 2007;59:445–53.
Singh S, Khera R, Allen AM, Murad MH, Loomba R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology. 2015;62:1417–32.
Vendemiale G, Grattagliano I, Caruso ML, Serviddio G, Valentini AM, Pirrelli M, et al. Increased oxidative stress in dimethylnitrosamine-induced liver fibrosis in the rat: effect of N-acetylcysteine and interferon-α. Toxicol Appl Pharmacol. 2001;175:130–9.
Al-Rasheed NM, Attia HA, Mohamad RA, Al-Rasheed NM, Al Fayez M, Al-Amin MA. Date fruits inhibit hepatocyte apoptosis and modulate the expression of hepatocyte growth factor, cytochrome P450 2E1 and heme oxygenase-1 in carbon tetrachloride-induced liver fibrosis. Arch Physiol Biochem. 2016;14:1–15.
Chiu HW, Hua KF. Hepatoprotective effect of wheat-based solid-state fermented Antrodia cinnamomea in carbon tetrachloride-induced liver injury in rat. PLoS One. 2016;11:e0153087.
Pulli B, Ali M, Iwamoto Y, Zeller MW, Schob S, Linnoila JJ, et al. Myeloperoxidase-hepatocyte-stellate cell cross talk promotes hepatocyte injury and fibrosis in experimental nonalcoholic steatohepatitis. Antioxid Redox Signal. 2015;23:1255–69.
Rensen SS, Bieghs V, Xanthoulea S, Arfianti E, Bakker JA, Shiri-Sverdlov R, et al. Neutrophil-derived myeloperoxidase aggravates non- alcoholic steatohepatitis in low-density lipoprotein receptor-deficient mice. PLoS One. 2012;7(12):e52411.
Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297–302.
van Dalen CJ, Winterbourn CC, Senthilmohan R, Kettle AJ. Nitrite as a substrate and inhibitor of myeloperoxidase. Implications for nitration and hypochlorous acid production at sites of inflammation. J Biol Chem. 2000;275:11638–44.
Doré S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA. 1999;96:2445–50.
Zeynalov E, Shah ZA, Li RC, Doré S. Heme oxygenase 1 is associated with ischemic preconditioning-induced protection against brain ischemia. Neurobiol Dis. 2009;35:264–9.
Lin X, Chen Y, Lv S, Tan S, Zhang S, Huang R, et al. Gypsophila elegans isoorientin attenuates CCl4-induced hepatic fibrosis in rats via modulation of NF-κB and TGF-β1/Smad signaling pathways. Int Immunopharmacol. 2015;28:305–12.
Mandal A, Bishayee A. Trianthema portulacastrum Linn. displays anti-inflammatory responses during chemically induced rat mammary tumorigenesis through simultaneous and differential regulation of NF-κB and Nrf2 signaling pathways. Int J Mol Sci. 2015;16:2426–45.
Surh YJ. NF-κB and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pac J Clin Nutr. 2008;17(Suppl 1):269–72.
Dreger H, Westphal K, Wilck N, Baumann G, Stangl V, Stangl K, et al. Protection of vascular cells from oxidative stress by proteasome inhibition depends on Nrf2. Cardiovasc Res. 2010;85:395–403.
Sengupta D, Chowdhury KD, Sarkar A, Paul S, Sadhukhan GC. Berberine and S allyl cysteine mediated amelioration of DEN + CCl4 induced hepatocarcinoma. Biochim Biophys Acta. 2014;1840:219–44.
Yang X, Yang S, Guo Y, Jiao Y, Zhao Y. Compositional characterisation of soluble apple polysaccharides, and their antioxidant and hepatoprotective effects on acute CCl4-caused liver damage in mice. Food Chem. 2013;138:1256–64.
Noh JR, Gang GT, Kim YH, Yang KJ, Hwang JH, Lee HS, et al. Antioxidant effects of the chestnut (Castanea crenata) inner shell extract in t-BHP-treated HepG2 cells, and CCl4- and high-fat diet-treated mice. Food Chem Toxicol. 2010;48:3177–83.
Reyes-Gordillo K, Segovia J, Shibayama M, Vergara P, Moreno MG, Muriel P. Curcumin protects against acute liver damage in the rat by inhibiting NF-κB, proinflammatory cytokines production and oxidative stress. Biochim Biophys Acta. 2007;1770:989–96.
Al-Rasheed NM, Fadda LM, Ali HM, Abdel Baky NA, El-Orabi NF, Al-Rasheed NM, et al. New mechanism in the modulation of carbon tetrachloride hepatotoxicity in rats using different natural antioxidants. Toxicol Mech Methods. 2016;26:243–50.
Acknowledgements
The authors extend their appreciation to the International Scientific Partnership Program at King Saud University for funding this research work through ISPP # 0084.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Alshammari, G.M., Balakrishnan, A. & Chinnasamy, T. 2-Hydroxy-4-methoxy benzoic acid attenuates the carbon tetra chloride-induced hepatotoxicity and its lipid abnormalities in rats via anti-inflammatory and antioxidant mechanism. Inflamm. Res. 66, 753–763 (2017). https://doi.org/10.1007/s00011-017-1054-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1054-2