Abstract
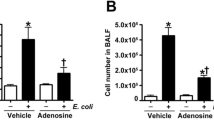
Neutrophil chemotaxis plays an essential role in recruiting neutrophils to sites of inflammation. Neutrophil chemotaxis is suppressed both after exposure to lipopolysaccharide (LPS) in vitro and during clinical and experimental endotoxemia, leading to serious consequences. Adenosine (ADO) is a potent anti-inflammatory agent that acts on a variety of neutrophil functions. However, its effects on human neutrophil chemotaxis during infection have been less well characterized. In the present study, we investigated the effect of ADO and its receptor-specific antagonist and agonist on neutrophil chemotaxis in an in vitro LPS-stimulated model. The results showed that increasing the concentration of ADO effectively restored the LPS-inhibited neutrophil chemotaxis to IL-8. A similar phenomenon occurred after intervention with a selective A1 receptor agonist but not with a selective antagonist. Pre-treatment with cAMP antagonist failed to restore LPS-inhibited chemotaxis. Furthermore, protein array and western blot analysis showed that the activation of A1 receptor significantly decreased LPS-induced p38 MAPK phosphorylation. However, the surface expression of the A1 receptor in LPS-stimulated neutrophils was not significantly changed. Taken together, these data indicated that ADO restored the LPS-inhibited chemotaxis via the A1 receptor, which downregulated the phosphorylation level of p38 MAPK, making this a promising new therapeutic strategy for infectious diseases.






Similar content being viewed by others
References
Wagner JG, Roth RA. Neutrophil migration during endotoxemia. J Leukoc Biol. 1999;66(1):10–24.
Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):856–64.
Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338(7):436–45.
Itoh Y, Okanoue T. Chemotactic cytokines (chemokines) in human hepatitis and experimental hepatitis models: which ones play the crucial role? J Gastroenterol. 2000;35(9):724–5.
Heit B, et al. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159(1):91–102.
Heit B, et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol. 2008;9(7):743–52.
Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6(7):541–50.
Maderazo EG, et al. Polymorphonuclear leukocyte migration abnormalities and their significance in seriously traumatized patients. Ann Surg. 1983;198(6):736–42.
Berger D, et al. Incidence and pathophysiological relevance of postoperative endotoxemia. FEMS Immunol Med Microbiol. 1995;11(4):285–90.
Matsuura M. Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front Immunol. 2013;4:109.
Putker F, Bos MP, Tommassen J. Transport of lipopolysaccharide to the Gram-negative bacterial cell surface. FEMS Microbiol Rev. 2015;39(6):985–1002.
Shimazu R, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189(11):1777–82.
Bohmer RH, Trinkle LS, Staneck JL. Dose effects of LPS on neutrophils in a whole blood flow cytometric assay of phagocytosis and oxidative burst. Cytometry. 1992;13(5):525–31.
Bishop NC, et al. Pre-exercise carbohydrate status and immune responses to prolonged cycling: I. Effect on neutrophil degranulation. Int J Sport Nutr Exerc Metab. 2001;11(4):490–502.
Guthrie LA, et al. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984;160(6):1656–71.
Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487(3):318–22.
Harkness RA, Simmonds RJ, Coade SB. Purine transport and metabolism in man: the effect of exercise on concentrations of purine bases, nucleosides and nucleotides in plasma, urine, leucocytes and erythrocytes. Clin Sci (Lond). 1983;64(3):333–40.
Bours MJ, et al. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404.
Hasko G, et al. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–70.
Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):247–64.
Fresco P, et al. Release inhibitory receptors activation favours the A2A-adenosine receptor-mediated facilitation of noradrenaline release in isolated rat tail artery. Br J Pharmacol. 2002;136(2):230–6.
Gessi S, et al. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117(1):123–40.
Quinn MT, DeLeo FR, Bokoch GM. Neutrophil methods and protocols. Preface. Methods Mol Biol. 2007;412:vii–viii.
Wang X, et al. Exogenous carbon monoxide inhibits neutrophil infiltration in LPS-induced sepsis by interfering with FPR1 via p38 MAPK but not GRK2. Oncotarget. 2016;7(23):34250–65.
Janetopoulos C, Firtel RA. Directional sensing during chemotaxis. FEBS Lett. 2008;582(14):2075–85.
Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278(23):20445–8.
Dilao R, Hauser MJ. Chemotaxis with directional sensing during Dictyostelium aggregation. C R Biol. 2013;336(11–12):565–71.
Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95(10):3032–43.
Perez-Aso M, et al. Adenosine A2A receptor and TNF-alpha regulate the circadian machinery of the human monocytic THP-1 cells. Inflammation. 2013;36(1):152–62.
Swain SD, et al. Inhibition of the neutrophil NADPH oxidase by adenosine is associated with increased movement of flavocytochrome b between subcellular fractions. Inflammation. 2003;27(1):45–58.
Salmon JE, Cronstein BN. Fc gamma receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy. A1 receptors are stimulatory and A2 receptors are inhibitory. J Immunol. 1990;145(7):2235–40.
van der Hoeven D, et al. A role for the low-affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils. J Pharmacol Exp Ther. 2011;338(3):1004–12.
McColl SR, et al. Immunomodulatory impact of the A2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J. 2006;20(1):187–9.
Inoue Y, et al. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30(2):173–7.
Jordan JE, et al. A(3) adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol. 1999;277(5 Pt 2):H1895–H905.
Foxman EF, Kunkel EJ, Butcher EC. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J Cell Biol. 1999;147(3):577–88.
McLeish KR, et al. Exocytosis of neutrophil granule subsets and activation of prolyl isomerase 1 are required for respiratory burst priming. J Innate Immun. 2013;5(3):277–89.
Rolas L, et al. Inhibition of mammalian target of rapamycin aggravates the respiratory burst defect of neutrophils from decompensated patients with cirrhosis. Hepatology. 2013;57(3):1163–71.
Marin V, et al. The p38 mitogen-activated protein kinase pathway plays a critical role in thrombin-induced endothelial chemokine production and leukocyte recruitment. Blood. 2001;98(3):667–73.
Pouliot M, et al. Expression and activity of prostaglandin endoperoxide synthase-2 in agonist-activated human neutrophils. FASEB J. 1998;12(12):1109–23.
Armstrong RA. Investigation of the inhibitory effects of PGE2 and selective EP agonists on chemotaxis of human neutrophils. Br J Pharmacol. 1995;116(7):2903–8.
Flamand N, et al. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol Pharmacol. 2002;62(2):250–6.
Flamand N, et al. Adenosine, a potent natural suppressor of arachidonic acid release and leukotriene biosynthesis in human neutrophils. Am J Respir Crit Care Med. 2000;161(2 Pt 2):S88–S94.
Cronstein BN, et al. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85(4):1150–7.
Cadieux JS, et al. Potentiation of neutrophil cyclooxygenase-2 by adenosine: an early anti-inflammatory signal. J Cell Sci. 2005;118(Pt 7):1437–47.
Pouliot M, et al. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol. 2002;169(9):5279–86.
Acknowledgements
This study was supported by the National Natural Science Foundation of China, No. 81071546, No. 81272148, No. 81171786, No. 81471903 and No. 81301657.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Bernhard Gibbs.
Rights and permissions
About this article
Cite this article
Xu, X., Zheng, S., Xiong, Y. et al. Adenosine effectively restores endotoxin-induced inhibition of human neutrophil chemotaxis via A1 receptor-p38 pathway. Inflamm. Res. 66, 353–364 (2017). https://doi.org/10.1007/s00011-016-1021-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-1021-3