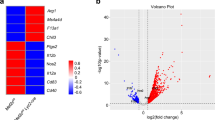
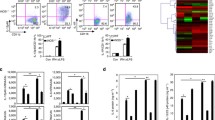
Abstract
Objective
Macrophages polarize to proinflammatory M1 or anti-inflammatory M2 states with distinct physiological functions. This transition within the M1–M2 phenotypes decides the nature, duration and severity of an inflammatory response. Although there is a substantial understanding of the fate of these phenotypes, the underlying molecular mechanism of transition within the M1–M2 phenotypes is not well understood. We have investigated the role of neuronal nitric oxide synthase (NOS1)-mediated regulation of activator protein 1 (AP-1) transcription factor in macrophages as a critical effector of macrophage phenotypic change.
Materials and Methods
Raw 264.7 and THP1 macrophages were stimulated with LPS (250 ng/ml) to activate the inflammatory signaling pathway. We analyzed the effect of pharmacological NOS1 inhibitor: TRIM (1-(2- Trifluoromethylphenyl) imidazole) on LPS-induced inflammatory response in macrophages.
Results
We determined that NOS1-derived nitric oxide (NO) facilitate Fos and Jun interaction which induces IL-12 & IL-23 expression. Pharmacological inhibition of NOS1 inhibits ATF2 and Jun dimer. Switching of Fos and Jun dimer to ATF2 and Jun dimerization controls phenotype transition from IL-12high IL-23high IL-10low to IL-12low IL-23lowIL-10high phenotype, respectively.
Conclusion
These findings highlight a key role of the TLR4-NOS1-AP1 signaling axis in regulating macrophage polarization.








Similar content being viewed by others
References
Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57.
Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, Mühl H. Macrophages in inflammation and its resolution. Inflammation 2012;3:324.
Jou I-M, Lin C-F, Tsai K-J, Wei S-J. Macrophage-mediated inflammatory disorders. Mediat Inflamm. 2013;2013:e316482.
Bashir S, Sharma Y, Elahi A, Khan F. Macrophage polarization: the link between inflammation and related diseases. Inflamm Res. 2015;65:1–11.
Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs functional differentiation. Front Immunol. 2014. doi:10.3389/fimmu.2014.00514.
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000 Prime Rep. 2014. doi:10.12703/P6-13.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95.
Liu Y-C, Zou X-B, Chai Y-F, Yao Y-M. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–9.
Mills CD. M1 and M2 Macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463–88.
Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol. 2014;5:614.
Günthner R, Anders H-J. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. 2013;2013:731023.
Rauch I, Müller M, Decker T. The regulation of inflammation by interferons and their STATs. JAK-STAT 2013;2(1):e23820.
Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, et al. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51:379–85.
Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:1135–44.
Fujimoto M, Naka T. SOCS1, a negative regulator of cytokine signals and TLR responses, in human liver diseases. Gastroenterol Res Pract. 2010;2010:470468. doi:10.1155/2010/470468
Wilson HM. SOCS proteins in macrophage polarization and function. Front Immunol. 2014;5. doi:10.3389/fimmu.2014.00357.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69.
Sabroe I, Parker L, Dower S, Whyte M. The role of TLR activation in inflammation. J Pathol. 2008;214:126–35.
Siegemund S, Sauer K. Balancing pro- and anti-inflammatory TLR4 signaling. Nat Immunol. 2012;13:1031–3.
Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61.
Lawrence T. The Nuclear factor NF-κB pathway in Inflammation. Cold Spring Harb Perspect Biol. 2009;1:6. doi:10.1101/cshperspect.a001651
Eräsalo H, Laavola M, Hämäläinen M, Leppänen T, Nieminen R, Moilanen E. PI3K inhibitors LY294002 and IC87114 reduce inflammation in carrageenan-induced paw oedema and down-regulate inflammatory gene expression in activated macrophages. Basic Clin Pharmacol Toxicol. 2015;116:53–61.
Hawkins PT, Stephens LR. PI3K signalling in inflammation. Biochim Biophys Acta BBA - Mol Cell Biol Lipids. 2015;1851:882–97.
Arranz A, Doxaki C, Vergadi E, Torre YM de la, Vaporidi K, Lagoudaki ED, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci. 2012;109:9517–22.
Couper KN, Blount DG, Riley EM. IL-10: The Master Regulator of Immunity to Infection. J Immunol. 2008;180:5771–7.
Gong D, Shi W, Yi S, Chen H, Groffen J, Heisterkamp N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31.
Mantovani Alberto, Sica Antonio, Locati M. Macrophage Polarization Comes of Age. Immunity. 2005;23:344–6.
Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, et al. NOS1-derived nitric oxide promotes NF-κB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med. 2015;212:1725–38.
Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, et al. Role of Cytokines as a Double-edged Sword in Sepsis. Vivo Athens Greece. 2013;27:669–84.
Zhang J-M, An J. Cytokines, Inflammation and Pain. Int Anesthesiol Clin. 2007;45:27–37.
Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J Leukoc Biol. 2010;88:1157–62.
Schulte W, Bernhagen J, Bucala R. Cytokines in Sepsis: Potent Immunoregulators and Potential Therapeutic Targets. Mediators Inflamm. 2013;2013:e165974.
Kröncke K-D, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113:147–56.
Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347–73.
Ramirez-Carrozzi V, Kerppola T. Asymmetric recognition of nonconsensus AP-1 sites by Fos-Jun and Jun-Jun influences transcriptional cooperativity with NFAT1. Mol Cell Biol. 2003;23:1737–49.
Yu T, Li YJ, Bian AH, Zuo HB, Zhu TW, Ji SX, et al. The regulatory role of activating transcription factor 2 in inflammation. Mediators Inflamm. 2014;2014:950472.
Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10:201.
Yang K, Wu Y, Xie H, Li M, Ming S, Li L, et al. Macrophage-mediated inflammatory response decreases mycobacterial survival in mouse MSCs by augmenting NO production. Sci Rep. 2016;6:27326.
Chinenov Y, Kerppola TK. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene. 2001;20:2438–52.
Lopez-Bergami P, Lau E, Ronai Z. ’ev. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat Rev Cancer. 2010;10:65–76.
Bogdan C. Of microbes, macrophages and nitric oxide. Behring Inst Mitt. 1997;58–72.
Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37.
MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50.
Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J Off Publ Fed Am Soc Exp Biol. 1992;6:3051–64.
Qu XW, Wang H, De Plaen IG, Rozenfeld RA, Hsueh W. Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15:439–46.
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7.
Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–44.
Malyshev I, Malyshev Y. Current concept and update of the macrophage plasticity concept: intracellular mechanisms of reprogramming and M3 macrophage "switch" phenotype. Biomed Res Int. 2015;2015:341308.
Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171–8.
Huang Z, Hoffmann FW, Fay JD, Hashimoto AC, Chapagain ML, Kaufusi PH, et al. Stimulation of unprimed macrophages with immune complexes triggers a low output of nitric oxide by calcium-dependent neuronal nitric-oxide synthase. J Biol Chem. 2012;287:4492–502.
Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–73.
Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–6.
Young MR, Li JJ, Rincón M, Flavell RA, Sathyanarayana BK, Hunziker R, et al. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci U S A. 1999;96:9827–32.
Wang A, Al-Kuhlani M, Johnston SC, Ojcius DM, Chou J, Dean D. Transcription factor complex AP-1 mediates inflammation initiated by Chlamydia pneumoniae infection. Cell Microbiol. 2013;15(5):779–94.
Acknowledgements
The authors are thankful to the Department of Biotechnology (DBT), Government of India-sponsored Ramalingaswami Fellowship to MSB. The authors also gratefully acknowledge the Indian Institute of Technology Indore for providing facilities and other support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding the contents of this article.
Additional information
Responsible Editor: John Di Battista.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Srivastava, M., Saqib, U., Naim, A. et al. The TLR4–NOS1–AP1 signaling axis regulates macrophage polarization. Inflamm. Res. 66, 323–334 (2017). https://doi.org/10.1007/s00011-016-1017-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-1017-z