Abstract
Objective
Systemic inflammatory response syndrome is associated with severe coagulopathy. The purpose of this study was to examine thrombin generation in systemic inflammation triggered by the endotoxin lipopolysaccharide (LPS) and the exotoxin streptococcal M1 protein.
Methods
Thrombin generation, lung histology and myeloperoxidase (MPO) activity were determined 6 and 24 h after induction of systemic inflammation. Male C57BL/6 mice received the Rac1 inhibitor NSC23766 prior to challenge with bacterial toxins.
Results
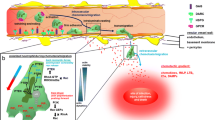
LPS and M1 protein challenge increased neutrophil infiltration and caused damage in the lung. Time to peak thrombin formation was increased and peak and total generation of thrombin were decreased in plasma from LPS- and M1 protein-treated mice. Coincubation of samples from mice exposed to bacterial toxins with platelet poor plasma from healthy mice completely reversed the inhibitory effect of LPS and M1 protein on thrombin generation, suggesting that bacterial toxins decreased levels of plasma factors explaining the reduction of thrombin generating capacity of plasma from septic animals. NSC23766 treatment not only decreased LPS- and M1 protein-induced neutrophil accumulation as well as levels of interleukin-6 and CXCL2 in the lung, but also abolished bacterial toxin-induced changes in thrombin generation. For example, NSC23766 increased peak formation by 57 % and total thrombin generation by 48 % in LPS-treated animals at 6 h.
Conclusions
Taken together, our novel findings show that bacterial toxins increase thrombin generation via consumption of plasma factors and that Rac1 signaling plays an important role in thrombin generation in response to bacterial toxins. Thus, targeting Rac1 activity might be a useful way not only to ameliorate pulmonary inflammation, but also inhibit pathological changes in coagulation in bacterial infections.






Similar content being viewed by others
References
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54.
Bochud PY, Glauser MP, Calandra T. International Sepsis F. Antibiotics in sepsis. Intensive Care Med. 2001;27(Suppl 1):S33–48.
Wang JE, Dahle MK, McDonald M, Foster SJ, Aasen AO, Thiemermann C. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock. 2003;20:402–14.
Bouza E, Finch R. Infections caused by Gram-positive bacteria: situation and challenges of treatment. Clin Microbiol Infect. 2001;7(Suppl 4):3.
Zhang S, Rahman M, Zhang S, Wang Y, Herwald H, Jeppsson B, et al. p38 Mitogen-activated protein kinase signaling regulates streptococcal M1 protein-induced neutrophil activation and lung injury. J Leukoc Biol. 2012;91:137–45.
Zhang S, Rahman M, Zhang S, Jeppsson B, Herwald H, Thorlacius H. Streptococcal m1 protein triggers farnesyltransferase-dependent formation of CXC chemokines in alveolar macrophages and neutrophil infiltration of the lungs. Infect Immun. 2012;80:3952–9.
Spero JA, Lewis JH, Hasiba U. Disseminated intravascular coagulation. Findings in 346 patients. Thromb Haemost. 1980;43:28–33.
Mann KG. Thrombin formation. Chest. 2003;124:4S–10S.
Shaw AD, Vail GM, Haney DJ, Xie J, Williams MD. Severe protein C deficiency is associated with organ dysfunction in patients with severe sepsis. J Crit Care. 2011;26:539–45.
Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost. 2011;9:1713–23.
Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence J, et al. Initial experiences with point-of-care rapid thrombelastography for management of life-threatening postinjury coagulopathy. Transfusion. 2012;52:23–33.
Kim JM, Oh YK, Lee JH, Im DY, Kim YJ, Youn J, et al. Induction of proinflammatory mediators requires activation of the TRAF, NIK, IKK and NF-kappaB signal transduction pathway in astrocytes infected with Escherichia coli. Clin Exp Immunol. 2005;140:450–60.
Mouawad F, Tsui H, Takano T. Role of Rho-GTPases and their regulatory proteins in glomerular podocyte function. Can J Physiol Pharmacol. 2013;91:773–82.
Hwaiz R, Hasan Z, Rahman M, Zhang S, Palani K, Syk I, et al. Rac1 signaling regulates sepsis-induced pathologic inflammation in the lung via attenuation of Mac-1 expression and CXC chemokine formation. J Surg Res. 2013;183:798–807.
Binker MG, Binker-Cosen AA, Gaisano HY, Cosen-Binker LI. Inhibition of Rac1 decreases the severity of pancreatitis and pancreatitis-associated lung injury in mice. Exp Physiol. 2008;93:1091–103.
Harada N, Iimuro Y, Nitta T, Yoshida M, Uchinami H, Nishio T, et al. Inactivation of the small GTPase Rac1 protects the liver from ischemia/reperfusion injury in the rat. Surgery. 2003;134:480–91.
Yao HY, Chen L, Xu C, Wang J, Chen J, Xie QM, et al. Inhibition of Rac activity alleviates lipopolysaccharide-induced acute pulmonary injury in mice. Biochim Biophys Acta. 2011;1810:666–74.
Luo L, Zhang S, Wang Y, Rahman M, Syk I, Zhang E, et al. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol Physiol. 2014;307:L586–96.
Wang Y, Roller J, Slotta JE, Zhang S, Luo L, Rahman M, et al. Distinct patterns of leukocyte recruitment in the pulmonary microvasculature in response to local and systemic inflammation. Am J Physiol Lung Cell Mol Physiol. 2013;304:L298–305.
Wang Y, Braun OO, Zhang S, Luo L, Norstrom E, Thorlacius H. Dynamic changes in thrombin generation in abdominal sepsis in mice. Shock. 2014;42:343–9.
Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Semi Thromb Hemost. 2013;39:559–66.
Picoli-Quaino SK, Alves BE, Faiotto VB, Montalvao SA, De Souza CA, Annichino-Bizzacchi JM, et al. Impairment of thrombin generation in the early phases of the host response of sepsis. J Crit Care. 2014;29:31–6.
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709.
Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–47.
Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32:249–53.
Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15.
Hasan Z, Palani K, Rahman M, Zhang S, Syk I, Jeppsson B, et al. Rho-kinase signaling regulates pulmonary infiltration of neutrophils in abdominal sepsis via attenuation of CXC chemokine formation and Mac-1 expression on neutrophils. Shock. 2012;37:282–8.
Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004.
Stefanini L, Boulaftali Y, Ouellette TD, Holinstat M, Desire L, Leblond B, et al. Rap1-Rac1 circuits potentiate platelet activation. Arterioscler Thromb Vasc Biol. 2012;32:434–41.
McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, et al. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474–84.
Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9:2251–61.
Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–61.
Pastuszczak M, Kotlarz A, Mostowik M, Zalewski J, Zmudka K, Undas A. Prior simvastatin treatment is associated with reduced thrombin generation and platelet activation in patients with acute ST-segment elevation myocardial infarction. Thromb Res. 2010;125:382–6.
Sanguigni V, Pignatelli P, Lenti L, Ferro D, Bellia A, Carnevale R, et al. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation. 2005;111:412–9.
Maddala RL, Reddy VN, Rao PV. Lovastatin-induced cytoskeletal reorganization in lens epithelial cells: role of Rho GTPases. Invest Ophthalmol Vis Sci. 2001;42:2610–5.
Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96:24F–33F.
Aslan JE, Baker SM, Loren CP, Haley KM, Itakura A, Pang J, et al. The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation. Am J Physiol Cell Physiol. 2013;305:C519–28.
Gadepalli R, Kotla S, Heckle MR, Verma SK, Singh NK, Rao GN. Novel role for p21-activated kinase 2 in thrombin-induced monocyte migration. J Biol Chem. 2013;288:30815–31.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (2012-3685), Yongzhi Wang is supported by a Grant from China Scholarship Council (2010631095).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Wang, Y., Hwaiz, R., Luo, L. et al. Rac1 regulates bacterial toxin-induced thrombin generation. Inflamm. Res. 65, 405–413 (2016). https://doi.org/10.1007/s00011-016-0924-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0924-3