Abstract
Objective
We have previously reported that myeloperoxidase-deficient (MPO−/−) neutrophils produce greater amounts of macrophage inflammatory protein-2 (MIP-2) upon in vitro stimulation with zymosan than wild-type neutrophils. This study aimed to examine the effect of MPO deficiency on the expression of other cytokines and chemokines.
Methods
Wild-type and MPO−/− neutrophils isolated from peritoneal cavity were stimulated with zymosan in vitro. Secretion of MIP-1α, MIP-1β, interleukin (IL)-1α, IL-1β, and tumor necrosis factor (TNF)-α by neutrophils was quantified by ELISA. mRNA expression in the neutrophils was analyzed by real-time reverse transcription-PCR, and the phosphorylation of extracellular-signal regulated kinase (ERK) 1/2 and p38 mitogen activated protein kinase (MAPK) in neutrophils was analyzed by western blot. For in vivo studies, mice were inoculated with zymosan intranasally, and the levels of these cytokines and chemokines were measured in the lungs.
Results
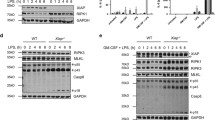
The MPO−/− neutrophils stimulated by zymosan expressed and secreted significantly higher levels of MIP-1α, MIP-1β, IL-1α, IL-1β, and TNF-α than the stimulated wild-type cells. Expression of all of these inflammatory mediators was blocked by pre-treatment with BAY11-7082, U0126, and SB203580, which are inhibitors of nuclear factor (NF)-κB, ERK1/2, and p38 MAPK, respectively. Enhanced expression of these inflammatory mediators is associated with elevated activation of ERK1/2 in stimulated MPO−/− neutrophils. In vivo, MPO−/− mice had significantly higher numbers of alveolar neutrophils and increased production of MIP-1α, MIP-1β, IL-1α, IL-1β, and TNF-α relative to the responses seen in wild-type mice within 24 h of zymosan administration.
Conclusion
MPO deficiency upregulates the expression of several proinflammatory cytokines and chemokines in mouse neutrophils.





Similar content being viewed by others
References
Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625.
Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93:185–98.
Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–36.
Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Ishida-Okawara A, et al. Contribution of the myeloperoxidase-dependent oxidative system to host defence against Cryptococcus neoformans. J Med Microbiol. 2006;55:1291–9.
Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, et al. Relative contributions of myeloperoxidase and NADPH-oxidase to the early host defense against pulmonary infections with Candida albicans and Aspergillus fumigatus. Med Mycol. 2002;40:557–63.
Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, et al. Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J Infect Dis. 2002;185:1833–7.
Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, et al. Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase. J Infect Dis. 2000;182:1276–9.
Takeuchi K, Umeki Y, Matsumoto N, Yamamoto K, Yoshida M, Suzuki K, et al. Severe neutrophil-mediated lung inflammation in myeloperoxidase-deficient mice exposed to zymosan. Inflamm Res. 2013;61:197–205.
Tateno N, Matsumoto N, Motowaki T, Suzuki K, Aratani Y. Myeloperoxidase deficiency induces MIP-2 production via ERK activation in zymosan-stimulated mouse neutrophils. Free Radic Res. 2013;47:376–85.
Homme M, Tateno N, Miura N, Ohno N, Aratani Y. Myeloperoxidase deficiency in mice exacerbates lung inflammation induced by nonviable Candida albicans. Inflamm Res. 2013;62:981–90.
De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180:4308–15.
Driscoll KE. TNFalpha and MIP-2: role in particle-induced inflammation and regulation by oxidative stress. Toxicol Lett. 2000;112–113:177–83.
Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–7.
Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–81.
Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25:469–84.
Apostolaki M, Armaka M, Victoratos P, Kollias G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr Dir Autoimmun. 2010;11:1–26.
Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–408.
Zampetaki A, Mitsialis SA, Pfeilschifter J, Kourembanas S. Hypoxia induces macrophage inflammatory protein-2 (MIP-2) gene expression in murine macrophages via NF-kappaB: the prominent role of p42/p44 and PI3 kinase pathways. FASEB J. 2004;18:1090–2.
Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–76.
Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–91.
Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–17.
Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–64.
Hatanaka E, Carvalho BT, Condino-Neto A, Campa A. Hyperresponsiveness of neutrophils from gp 91phox deficient patients to lipopolysaccharide and serum amyloid A. Immunol Lett. 2004;94:43–6.
Lekstrom-Himes JA, Kuhns DB, Alvord WG, Gallin JI. Inhibition of human neutrophil IL-8 production by hydrogen peroxide and dysregulation in chronic granulomatous disease. J Immunol. 2005;174:411–7.
Brown KL, Bylund J, MacDonald KL, Song-Zhao GX, Elliott MR, Falsafi R, et al. ROS-deficient monocytes have aberrant gene expression that correlates with inflammatory disorders of chronic granulomatous disease. Clin Immunol. 2008;129:90–102.
Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–66.
Cassatella MA. Interferon-gamma inhibits the lipopolysaccharide-induced macrophage inflammatory protein-1 alpha gene transcription in human neutrophils. Immunol Lett. 1996;49:79–82.
Fernandez MC, Walters J, Marucha P. Transcriptional and post-transcriptional regulation of GM-CSF-induced IL-1 beta gene expression in PMN. J Leukoc Biol. 1996;59:598–603.
Chen BC, Chang YS, Kang JC, Hsu MJ, Sheu JR, Chen TL, et al. Peptidoglycan induces nuclear factor-kappaB activation and cyclooxygenase-2 expression via Ras, Raf-1, and ERK in RAW 264.7 macrophages. J Biol Chem. 2004;279:20889–97.
Guha M, O’Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, et al. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–39.
Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75.
Carter AB, Monick MM, Hunninghake GW. Both Erk and p38 kinases are necessary for cytokine gene transcription. Am J Respir Cell Mol Biol. 1999;20:751–8.
Cloutier A, Ear T, Blais-Charron E, Dubois CM, McDonald PP. Differential involvement of NF-kappaB and MAP kinase pathways in the generation of inflammatory cytokines by human neutrophils. J Leukoc Biol. 2007;81:567–77.
Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y, et al. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–82.
Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014;26:237–45.
Hamilton TA, Novotny M, Datta S, Mandal P, Hartupee J, Tebo J, et al. Chemokine and chemoattractant receptor expression: post-transcriptional regulation. J Leukoc Biol. 2007;82:213–9.
Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–74.
Nick JA, Avdi NJ, Young SK, Lehman LA, McDonald PP, Frasch SC, et al. Selective activation and functional significance of p38alpha mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J Clin Invest. 1999;103:851–8.
Dai Y, Datta S, Novotny M, Hamilton TA. TGFbeta inhibits LPS-induced chemokine mRNA stabilization. Blood. 2003;102:1178–85.
Hamilton T, Novotny M, Pavicic PJ Jr, Herjan T, Hartupee J, Sun D, et al. Diversity in post-transcriptional control of neutrophil chemoattractant cytokine gene expression. Cytokine. 2010;52:116–22.
Acknowledgments
This work was supported in part by JSPS KAKENHI Grant number 26450446.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Additional information
Responsible Editor: Bernhard Gibbs.
D. Endo and T. Saito contributed equally to this work and are joint first authors.
Rights and permissions
About this article
Cite this article
Endo, D., Saito, T., Umeki, Y. et al. Myeloperoxidase negatively regulates the expression of proinflammatory cytokines and chemokines by zymosan-induced mouse neutrophils. Inflamm. Res. 65, 151–159 (2016). https://doi.org/10.1007/s00011-015-0899-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0899-5