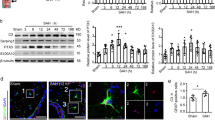
Abstract
Objective
The aim of this study was to investigate whether fimasartan, a novel angiotensin II receptor blocker, modulates hemolysate-induced inflammation in astrocytes.
Methods
We stimulated astrocytes with hemolysate to induce hemorrhagic inflammation in vitro. Astrocytes were pretreated with fimasartan and then incubated with hemolysate at different durations. Anti-inflammatory cell signaling molecules including Akt, extracellular signal regulated kinase (ERK), NFκB and cyclooxygenase-2 (COX-2) were assessed by western blotting. Pro-inflammatory mediators were evaluated by real-time RT-PCR and ELISA.
Results
The stimulation by hemolysate generated a robust activation of inflammatory signaling pathways in astrocytes. Hemolysate increased the phosphorylation of Akt at 1 h, and ERK1/2 at 20 min compared with the control group and promoted the degradation of IκBα. Pretreated fimasartan significantly decreased hemolysate-induced phosphorylation of Akt and ERK1/2. In addition, fimasartan also suppressed NFκB-related inflammatory pathways induced by hemolysate, including reduction of the gene expression of NFκB, and decreased nuclear translocation of NFκB and degradation of IκB. This reduction of inflammatory upstream pathways decreased the expression of inflammatory end-products: COX-2 and interleukin-1 (IL-1β). Furthermore, the expression of COX-2 was attenuated by both Akt inhibitor (LY294002) and ERK inhibitor (U0126), and IκBα degradation was suppressed by LY294002.
Conclusions
These results demonstrate that pretreatment with fimasartan to astrocytes suppresses the inflammatory responses induced by hemolysate. Akt, ERK and NFκB were associated with hemolysate-induced COX-2 and IL-1β expression. Based on these mechanisms, fimasartan could be a candidate anti-inflammatory regulator for the treatment of intracerebral hemorrhage.





Similar content being viewed by others
References
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44.
Lee ST, Chu K, Sinn DI, Jung KH, Kim EH, Kim SJ, et al. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. J Neurochem. 2006;96:1728–39.
Hong K-S, Bang OY, Kang D-W, Yu K-H, Bae H-J, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the korean stroke society and clinical research center for stroke. J Stroke. 2013;15:2–20.
Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;26:230–52.
Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63.
Sasaki T, Kasuya H, Onda H, Sasahara A, Goto S, Hori T, et al. Role of p38 mitogen-activated protein kinase on cerebral vasospasm after subarachnoid hemorrhage. Stroke. 2004;35:1466–70.
Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. 2001;32:2932–8.
Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–82 (discussion 882–3).
Lu H, Shi J-X, Zhang D-M, Shen J, Lin Y-X, Hang C-H, et al. Hemolysate-induced expression of intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 expression in cultured brain microvascular endothelial cells via through ros-dependent nf-κb pathways. Cell Mol Neurobiol. 2009;29:87–95.
Edye ME, Lopez-Castejon G, Allan SM, Brough D. Acidosis drives damage-associated molecular pattern (DAMP)-induced interleukin-1 secretion via a caspase-1-independent pathway. J Biol Chem. 2013;288:30485–94.
Lu H, Shi JX, Zhang DM, Wang HD, Hang CH, Chen HL, et al. Inhibition of hemolysate-induced iNOS and COX-2 expression by genistein through suppression of NF-small ka, CyrillicB activation in primary astrocytes. J Neurol Sci. 2009;278:91–5.
Lu H, Shi J-X, Zhang D-M, Chen H-L, Qi M, Yin H-X. Genistein, a soybean isoflavone, reduces the production of pro-inflammatory and adhesion molecules induced by hemolysate in brain microvascular endothelial cells. Acta Neurol Belg. 2009;109:32.
Zanchetti A, Elmfeldt D. Findings and implications of the study on cognition and prognosis in the elderly (SCOPE)–a review. Blood Press. 2006;15:71–9.
McFarlane SI. Role of angiotensin receptor blockers in diabetes: implications of recent clinical trials. Expert Rev Cardiovasc Ther. 2009;7:1363–71.
Ismail H, Mitchell R, McFarlane SI, Makaryus AN. Pleiotropic effects of inhibitors of the RAAS in the diabetic population: above and beyond blood pressure lowering. Curr Diabetes Rep. 2010;10:32–6.
Benicky J, Sánchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, et al. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36:857–70.
Lou M, Blume A, Zhao Y, Gohlke P, Deuschl G, Herdegen T, et al. Sustained blockade of brain AT1 receptors before and after focal cerebral ischemia alleviates neurologic deficits and reduces neuronal injury, apoptosis, and inflammatory responses in the rat. J Cereb Blood Flow Metab. 2004;24:536–47.
Faure S, Oudart N, Javellaud J, Fournier A, Warnock DG, Achard J-M. Synergistic protective effects of erythropoietin and olmesartan on ischemic stroke survival and post-stroke memory dysfunctions in the gerbil. J Hypertens. 2006;24:2255–61.
Saavedra JM, Angiotensin II. AT1 receptor blockers as treatments for inflammatory brain disorders. Clin Sci Lond. 2012;123:567–90.
Villapol S, Saavedra JM. Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens. 2015;28:289–99.
Sanchez-Lemus E, Murakami Y, Larrayoz-Roldan IM, Moughamian AJ, Pavel J, Nishioku T, et al. Angiotensin II AT1 receptor blockade decreases lipopolysaccharide-induced inflammation in the rat adrenal gland. Endocrinology. 2008;149:5177–88.
Kono S, Kurata T, Sato K, Omote Y, Hishikawa N, Yamashita T, et al. Neurovascular protection by telmisartan via reducing neuroinflammation in stroke-resistant spontaneously hypertensive rat brain after ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:537–47.
Jung K-H, Chu K, Lee S-T, Kim S-J, Song E-C, Kim E-H, et al. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J Pharmacol Exp Ther. 2007;322:1051–8.
Kim JH, Lee JH, Paik SH, Kim JH, Chi YH. Fimasartan, a novel angiotensin II receptor antagonist. Arch Pharm Res. 2012;35:1123–6.
Han J, Park SJ, Thu VT, Lee SR, le Long T, Kim HK, et al. Effects of the novel angiotensin II receptor type I antagonist, fimasartan on myocardial ischemia/reperfusion injury. Int J Cardiol. 2013;168:2851–9.
Kim CK, Yang X-L, Kim Y-J, Choi I-Y, Jeong H-G, Park H-K, et al. Effect of long-term treatment with fimasartan on transient focal ischemia in rat brain. Biomed Res Int. 2015;2015:295925. doi:10.1155/2015/295925.
Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci Lond. 2007;112:375–84.
Aoki T, Takenaka K, Suzuki S, Kassell NF, Sagher O, Lee KS. The role of hemolysate in the facilitation of oxyhemoglobin-induced contraction in rabbit basilar arteries. J Neurosurg. 1994;81:261–6.
Fann DY, Santro T, Manzanero S, Widiapradja A, Cheng YL, Lee SY, et al. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp Neurol. 2014;257:114–9.
Deroide N, Li X, Lerouet D, Van Vré E, Baker L, Harrison J, et al. MFGE8 inhibits inflammasome-induced IL-1β production and limits postischemic cerebral injury. J Clin Invest. 2013;123:1176.
Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–52.
Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42:1781–6.
Benicky J, Sanchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol. 2009;29:781–92.
Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev. 2013;65:809–48.
Shimizu K, Takashima T, Yamane T, Sasaki M, Kageyama H, Hashizume Y, et al. Whole-body distribution and radiation dosimetry of [11C] telmisartan as a biomarker for hepatic organic anion transporting polypeptide (OATP) 1B3. Nucl Med Biol. 2012;39:847–53.
Noda A, Fushiki H, Murakami Y, Sasaki H, Miyoshi S, Kakuta H, et al. Brain penetration of telmisartan, a unique centrally acting angiotensin II type 1 receptor blocker, studied by PET in conscious rhesus macaques. Nucl Med Biol. 2012;39:1232–5.
Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90.
Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, et al. Angiotensin II sustains brain inflammation in mice via TGF-β. J Clin Invest. 2010;120:2782.
Saavedra JM. Brain and pituitary angiotensin. Endocr Rev. 1992;13:329–80.
Trendelenburg G. Molecular regulation of cell fate in cerebral ischemia: role of the inflammasome and connected pathways. J Cereb Blood Flow Metab. 2014;34:1857–67.
Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–45.
Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell. 2005;37:289–305.
Zhao Y, Rempe DA. Targeting astrocytes for stroke therapy. Neurotherapeutics. 2010;7:439–51.
Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a chinese cohort study. Arthritis Rheum. 2009;61:225–32.
Shih VF-S, Tsui R, Caldwell A, Hoffmann A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011;21:86–102.
Novack DV. Role of NF-κB in the skeleton. Cell Res. 2011;21:169–82.
Xu C, Shen G, Chen C, Gélinas C. Kong A-NT. Suppression of NF-κB and NF-κB-regulated gene expression by sulforaphane and PEITC through IκBα, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95.
O’Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–8.
Ryu S, Shin JS, Cho YW, Kim HK, Paik SH, Lee JH, et al. Fimasartan, anti-hypertension drug, suppressed inducible nitric oxide synthase expressions via nuclear factor-kappa B and activator protein-1 inactivation. Biol Pharm Bull. 2013;36:467–74.
Acknowledgments
This work was supported by grant from the Boryung Pharmaceutical Company (800-20150256), and the Health Fellowship Foundation (2014-810115), Seoul, Republic of Korea. The research was also supported by a grant of the Korean Health Technology R&D Project funded by the Ministry of Health & Welfare, Republic of Korea (HI14C1277). The funding organization had no role in the design, conduct, or analysis conducted during this study, or in the preparation of this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have nothing to disclose.
Additional information
Responsible Editor: Ji Zhang.
X.- L. Yang and C. K. Kim equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, XL., Kim, C.K., Kim, T.J. et al. Anti-inflammatory effects of fimasartan via Akt, ERK, and NFκB pathways on astrocytes stimulated by hemolysate. Inflamm. Res. 65, 115–123 (2016). https://doi.org/10.1007/s00011-015-0895-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0895-9