Abstract
Objective
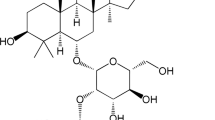
The present study aims to explore the effects of paeoniflorin (PF), a monoterpene glycoside isolated from the roots of Paeonia lactiflora Pallas, on acute lung injury (ALI) and the possible mechanisms.
Materials and method
ALI was induced in mice by an intratracheal instillation of lipopolysaccharide (LPS, 1 mg/kg), and PF was injected intraperitoneally 30 min prior to LPS administration. After 24 h, lung water content, histology, microvascular permeability and proinflammatory cytokines in the bronchoaveolar lavage fluid were evaluated.
Results
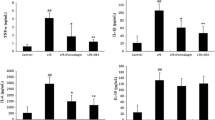
It was shown that PF (50, 100 mg/kg) could alleviate LPS-induced ALI, evidenced by reduced pulmonary edema, improved histological changes, and attenuated inflammatory cell accumulation in the interstitium and alveolar space as well as microvascular permeability. It also markedly down-regulated the expressions of proinflammatory cytokines interleukin (IL)-1β and tumor necrosis factor (TNF)-α at both transcription and protein levels. Additionally, PF inhibited the phosphorylations of p38 MAP kinase (p38) and c-Jun NH2-terminal kinase (JNK) but not extracellular signal-regulated kinase (ERK), and prevented the activation of nuclear factor-kappa B (NF-κB) in the lung tissues.
Conclusion
The findings suggest that PF is able to alleviate ALI, and the underlying mechanisms are probably attributed to decreasing the production of proinflammatory cytokines through down-regulation of the activation of p38, JNK and NF-κB pathways in lung tissues.







Similar content being viewed by others
References
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93.
Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol. 2007;179:1254–63.
Ngamsri KC, Wagner R, Vollmer I, Stark S, Reutershan J. Adenosine receptor A1 regulates polymorphonuclear cell trafficking and microvascular permeability in lipopolysaccharide-induced lung injury. J Immunol. 2010;185:4374–84.
Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–15.
Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–57.
Jain R, DalNogare A. Pharmacological therapy for acute respiratory distress syndrome. Mayo Clin Proc. 2006;81:205–12.
Lee B, Shin YW, Bae EA, Han SJ, Kim JS, Kang SS, et al. Antiallergic effect of the root of Paeonia lactiflora and its constituents paeoniflorin and paeonol. Arch Pharm Res. 2008;31:445–50.
Zheng YQ, Wei W, Zhu L, Liu JX. Effects and mechanisms of Paeoniflorin, a bioactive glucoside from paeony root, on adjuvant arthritis in rats. Inflamm Res. 2007;56:182–8.
Liu DF, Wei W, Song LH. Protective effect of paeoniflorin on immunological liver injury induced by bacillus Calmette-Guerin plus lipopolysaccharide: modulation of tumour necrosis factor-alpha and interleukin-6 MRNA. Clin Exp Pharmacol Physiol. 2006;33:332–9.
Yu HY, Liu MG, Liu DN, Shang GW, Wang Y, Qi C, et al. Antinociceptive effects of systemic paeoniflorin on bee venom-induced various ‘phenotypes’ of nociception and hypersensitivity. Pharmacol Biochem Behav. 2007;88:131–40.
Jiang WL, Chen XG, Zhu HB, Gao YB, Tian JW, Fu FH. Paeoniflorin inhibits systemic inflammation and improves survival in experimental sepsis. Basic Clin Pharmacol Toxicol. 2009;105:64–71.
Kim ID, Ha BJ. The effects of paeoniflorin on LPS-induced liver inflammatory reactions. Arch Pharm Res. 2010;33:959–66.
Xu H, Gao XH, Song J, Wang FY, Xu Z, Lu D, et al. Peoniflorin prevents the adhesion between inflammatory endothelial cells and leukocytes through inhibiting the activation of MAPKs and NF-κB. Drug Dev Res. 2010;71:275–84.
Zhang XJ, Chen HL, Li Z, Zhang HQ, Xu HX, Sung JJ, et al. Analgesic effect of paeoniflorin in rats with neonatal maternal separation-induced visceral hyperalgesia is mediated through adenosine A(1) receptor by inhibiting the extracellular signal-regulated protein kinase (ERK) pathway. Pharmacol Biochem Behav. 2009;94:88–97.
Liu HQ, Zhang WY, Luo XT, Ye Y, Zhu XZ. Paeoniflorin attenuates neuroinflammation and dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease by activation of adenosine A1 receptor. Br J Pharmacol. 2006;148:314–25.
Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E. Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol. 2007;179:7079–86.
Jacobson JR, Barnard JW, Grigoryev DN, Ma SH, Tuder RM, Garcia JGN. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–32.
Zhang XM, Song KJ, Xiong HZ, Li HY, Chu X, Deng XM. Protective effect of florfenicol on acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol. 2009;9:1525–9.
Tan ZH, Yu LH, Wei HL, Liu GT. Scutellarin protects against lipopolysaccharide-induced acute lung injury via inhibition of NF-kappaB activation in mice. J Asian Nat Prod Res. 2010;12:175–84.
Liu S, Feng G, Wang GL, Liu GJ. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol. 2008;584:159–65.
Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–99.
Tumurkhuu G, Koide N, Dagvadorj J, Morikawa A, Hassan F, Islam S, et al. The mechanism of development of acute lung injury in lethal endotoxic shock using alpha-galactosylceramide sensitization. Clin Exp Immunol. 2008;152:182–91.
Wang HD, Lu DX, Qi RB. Therapeutic strategies targeting the LPS signaling and cytokines. Pathophysiology. 2009;16:291–6.
Liaudet L, Mabley JG, Pacher P, Virág L, Soriano FG, Marton A, et al. Inosine exerts a broad range of antiinflammatory effects in a murine model of acute lung injury. Ann Surg. 2002;235:568–78.
Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, et al. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J. 2002;16:1713–20.
Asti C, Ruggieri V, Porzio S, Chiusaroli R, Melillo G, Caselli GF. Lipopolysaccharide-induced lung injury in mice. I. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage. Pulm Pharmacol Ther. 2000;13:61–9.
Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods. 1997;202:49–57.
Camp SM, Bittman R, Chiang ET, Moreno-Vinasco L, Mirzapoiazova T, Sammani S, et al. Synthetic analogs of FTY720 [2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol] differentially regulate pulmonary vascular permeability in vivo and in vitro. J Pharmacol Exp Ther. 2009;331:54–64.
Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–56.
Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F 2nd, Park DR, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–903.
Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, et al. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ Res. 2008;102:804–12.
Cao WJ, Zhang W, Liu JJ, Wang Y, Peng XM, Lu DX, et al. Paeoniflorin improves survival in LPS-challenged mice through the suppression of TNF-α and IL-1β release and augmentation of IL-10 production. Int Immunopharmacol. 2011;11:172–8.
Walley KR, McDonald TE, Higashimoto Y, Hayashi S. Modulation of proinflammatory cytokines by nitric oxide in murine acute lung injury. Am J Respir Crit Care Med. 1999;160:698–704.
Mehta S. The effects of nitric oxide in acute lung injury. Vasc Pharmacol. 2005;43:390–403.
Wang LF, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, et al. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med. 2002;165:1634–9.
Mikawa K, Nishina K, Takao Y, Obara H. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg. 2003;97:1751–5.
Yoshihisa Y, Furuichi M, Urrehman M, Ueda C, Makino T, Shimizu T. The traditional Japanese formula keishibukuryogan inhibits the production of inflammatory cytokines by dermal endothelial cells. Mediat Inflamm. 2010;2010:1–8.
Sun Y, Dong Y, Jiang HJ, Cai TT, Chen L, Zhou X, et al. Dissection of the role of paeoniflorin in the traditional Chinese medicinal formula Si-Ni-San against contact dermatitis in mice. Life Sci. 2009;84:337–44.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20.
Kim HJ, Lee HS, Chong YH, Kang JL. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB activation in the development of lung injury and RAW 264.7 macrophages. Toxicology. 2006;225:36–47.
Schuh K, Pahl A. Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury. Biochem Pharmacol. 2009;77:1827–34.
Chen T, Guo ZP, Jiao XY, Jia RZ, Zhang YH, Li JY, et al. Paeoniflorin suppresses tumor necrosis factor-α induced chemokineproduction in human dermal microvascular endothelial cells by blocking nuclear factor-κB and ERK pathway. Arch Dermatol Res. 2011; 303:351-360.
Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20.
Jin L, Zhang LM, Xie KQ, Ye Y, Feng L. Paeoniflorin suppresses the expression of ICAM-1 in endotoxin-treated human monocytic cells. Br J Pharmacol. 2011. doi:10.1111/j.1476-5381.2011.01464.x.
Wu H, Li W, Wang T, Shu Y, Liu P. Paeoniflorin suppress NF-kappaB activation through modulation of I kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human gastric carcinoma cells. Biomed Pharmacother. 2008;62:659–66.
Asai M, Kawashima D, Katagiri K, Takeuchi R, Tohnai G, Ohtsuka K. Protective effect of a molecular chaperone inducer, paeoniflorin, on the HCl- and ethanol-triggered gastric mucosal injury. Life Sci. 2010;88:350–7.
Fu J, Li Y, Wang L, Gao B, Zhang N, Ji Q. Paeoniflorin prevents diabetic nephropathy in rats. Comp Med. 2009;59:557–66.
Acknowledgments
This work was supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and partially supported by Innovative Training Plan for Undergraduate Students of China Pharmaceutical University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael Parnham.
Rights and permissions
About this article
Cite this article
Zhou, H., Bian, D., Jiao, X. et al. Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviating inflammatory cell infiltration and microvascular permeability. Inflamm. Res. 60, 981–990 (2011). https://doi.org/10.1007/s00011-011-0359-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0359-9