Abstract
Background
Parenteral nutrition is an important risk factor for late onset sepsis in neonates. This may be caused by the long-term need of central venous access but also through a potentially modulating effect of lipids and glucose on the immune function.
Objective
It was the aim of this study to characterize the effect of lipids and glucose on the neonatal immune response in an in vitro Staphylococcus epidermidis sepsis model using whole cord blood of healthy term infants and preterm infants.
Results
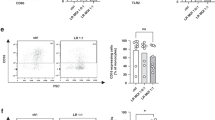
At the single cell level, IL-6, IL-8 and TNF-α expression of CD14+ cells was significantly increased upon addition of 1% lipids, while the addition of clinically meaningful lipid concentrations had no remarkable effect. When glucose was added to whole cord blood cultures, a dose-dependent effect was demonstrated for IL-8 expression but not for other cytokines.
Conclusions
These in vitro data suggest that the pro-inflammatory cytokine response to S. epidermidis may be modulated by lipids and glucose. Further studies are needed to investigate whether these findings are applicable to clinical settings and to evaluate the role of cytokine monitoring in infants receiving long-term parenteral nutrition.



Similar content being viewed by others
Abbreviations
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- S. epidermidis :
-
Staphylococcus epidermidis
References
Okada Y, Klein NJ, von Saene HK, Webb G, Holzel H, Pierro A. Bactericidal activity against coagulase-negative staphylococci is impaired in infants receiving long-term parenteral nutrition. Ann Surg. 2000;231:276–81.
Dinerstein A, Nieto RM, Solana CL, Perez GP, Otheguy GP, Largia AM. Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants. J Perinatol. 2006;26:436–42.
Bohles H. Indications for lipid infusion in pediatric patients. Klin Padiatr. 1989;201:146–53.
Lai NM, Rajadurai SV, Tan KH. Increased energy intake for preterm infants with (or developing) bronchopulmonary dysplasia/chronic lung disease. Cochrane Database Syst Rev 2006; 3: CD005093.
Wesley JR, Coran AG. Intravenous nutrition for the pediatric patient. Semin Pediatr Surg. 1992;1:212–30.
Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301.
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91.
Marchini G, Lindow S, Brismar H, Ståbi B, Berggren V, Ulfgren AK, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. 2002;147:1127–34.
Costa SF, Miceli MH, Anaissie EH. Mucosa or skin as source of coagulase-negative staphylococcal bacteraemia. Lancet Infect Dis. 2004;4:278–86.
Jarlov JO. Phenotypic characteristics of coagulase-negative staphylococci: typing and antibiotic susceptibility. APMIS Suppl. 1999;91:1–42.
Avila-Figueroa C, Goldmann DA, Richardson DK, Gray JE, Ferrari A, Freeman J. Intravenous lipid emulsions are the major determinant of coagulase-negative staphylococcal bacteremia in very low birth weight newborns. Pediatr Infect Dis J. 1998;17:10–7.
Sweeney B, Puri B, Reen DJ. Polyunsaturated fatty acids influence neonatal monocyte survival. Pediatr Surg Int. 2001;17:254–8.
Sweeney B, Puri B, Reen DJ. Modulation of immune cell function by polyunsaturated fatty acids. Pediatr Surg Int. 2005;21:335–40.
Yu WK, Li WQ, Li N, Li JS. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol. 2003;9:1824–7.
Monson JR, Ramsden CW, MacFie J, Brennan TG, Guillou PJ. Immunorestorative effect of lipid emulsions during total parenteral nutrition. Br J Surg. 1986;73:843–6.
Sedman PC, Somers SS, Ramsden CW, Brennan TG, Guillou PJ. Effects of different lipid emulsions on lymphocyte function during total parenteral nutrition. Br J Surg. 1991;78:1396–9.
Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31:359–66.
Calder PC. Effects of fatty acids and dietary lipids on cells of the immune system. Proc Nutr Soc. 1996;55:127–50.
Calder PC. N-3 polyunsaturated fatty acids and immune cell function. Adv Enzyme Regul. 1997;37:197–237.
Hartel C, et al. Characterisation of the host inflammatory response to Staphylococcus epidermidis in neonatal whole blood. Arch Dis Child Fetal Neonatal Ed. 2008;93:F140–5.
Janeway CA. Immunobiology. 6th ed. New York: Taylor & Francis Group; 2005:76.
Bjorkqvist M, et al. Phenotypic and genotypic characterisation of blood isolates of coagulase-negative staphylococci in the newborn. APMIS. 2002;110(4):332–9.
Härtel C, Osthues I, Rupp J, Haase B, Röder K, Göpel W, et al. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? J Pediatr Gastroenterol Nutr. 2009;48:464–70.
Sweeney B, Puri B, Reen DJ. Induction and modulation of apoptosis in neonatal monocytes by polyunsaturated fatty acids. J Pediatr Surg. 2007;42:620–8.
Fischer GW, Hunter KW, Wilson SR, Mease AD. Diminished bacterial defences with intralipid. Lancet. 1980;2:819–20.
Härtel C, Schultz C, Herting E, Göpel W. Genetic association studies in VLBW infants exemplifying susceptibility to sepsis—recent findings and implications for future research. Acta Paediatr. 2007;96:158–65.
Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123:1314–9.
Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118:1811–8.
Lilien LD, Rosenfield RL, Baccaro MM, Pildes RS. Hyperglycemia in stressed small premature neonates. J Pediatr. 1979;94:454–9.
Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533–51.
Mizock BA. Blood glucose management during critical illness. Rev Endocr Metab Disord. 2003;4:187–94.
Losser MR, Bernard C, Beaudeux JL, Pison C, Payen D. Glucose modulates hemodynamic, metabolic, and inflammatory responses to lipopolysaccharide in rabbits. J Appl Physiol. 1997;83:1566–74.
Reinhold D, Ansorge S, Schleicher ED. Elevated glucose levels stimulate transforming growth factor-beta 1 (TGF-beta 1), suppress interleukin IL-2, IL-6 and IL-10 production and DNA synthesis in peripheral blood mononuclear cells. Horm Metab Res. 1996;28:267–70.
Gregory R, McElveen J, Tattersall RB, Todd I. The effects of 3-hydroxybutyrate and glucose on human T cell responses to Candida albicans. FEMS Immunol Med Microbiol. 1993;7:315–20.
Matsukawa A, Hogaboam CM, Lukacs NW, Kunkel SL. Chemokines and innate immunity. Rev Immunogenet. 2000;2:339–58.
Yaqoob P. Monosaturated fatty acids in parenteral nutrition, evaluation of risks and benefits. Br J Nutr. 2005;94:867–8.
Acknowledgments
We thank Anja Sewe for excellent technical support. This study was supported by University of Lübeck Research Grants (BH, CH).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Haase, B., Faust, K., Heidemann, M. et al. The modulatory effect of lipids and glucose on the neonatal immune response induced by Staphylococcus epidermidis . Inflamm. Res. 60, 227–232 (2011). https://doi.org/10.1007/s00011-010-0258-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0258-5