Abstract
The development of new biomaterials and medical devices has become a growing field of interdisciplinary research. The medical devices for tissue and cell treatments are being constructed for the application in regenerative medicine. There are many different approaches to improve cellular functions and it is known that physical stimuli affect cell physiology such as proliferation and differentiation. In this review we focus on electrical and mechanical stimulation as well as cold atmospheric pressure plasma treatment and photobiomodulation. Bone forming cells show improved proliferation and migration after electrical stimulation, which is used as treatment in bone fracture healing and to enhance osseointegration. Especially mechanical forces have direct effects on central cell signalling pathways and cell adhesion to biomaterial surfaces. Physical plasma promotes tissue regeneration and exhibits anti-carcinogenic effects, while light of different wavelengths also improves wound healing and tissue repair by influencing stem cell fate. Although the treatment approaches are different, all these physical factors lead to the activation of cell signalling via calcium and reactive oxygen species. A better understanding of the cellular response to the applied stimuli will help develop efficient treatment strategies and optimised device settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical stimuli are essential for cell functions as well as important players in normal tissue development, cell regeneration, and disease. Just as organisms can sense their environment, individual cells recognise physical parameters in the extracellular environment. For optimal growth of cells in vitro, the pH, temperature, and CO2 concentration must be controlled and adapted to fit the cell’s needs. Apart from these well-known and established parameters many other physical stimuli influence cellular behaviour. The stiffness of the surface, on which cells grow, influences their phenotype and gene expression [1]. For example, stem cells differentiate in a osteoblastic direction when grown on rigid surfaces, while stem cells grown on softer substrates favour adipogenic differentiation [2, 3]. The transduction of extracellular mechanical forces into intracellular biochemical signals is called mechanotransduction, which is also responsible for translating shear stress or other mechanical stimuli into cellular signals. The variety of physical stimuli and the complexity of extra- and intracellular signals triggered are very large. However, second messengers such as calcium ions (Ca2+) and reactive oxygen species (ROS) play important roles in the activation of downstream signalling. Ca2+ concentrations in the cytoplasm are ca. 20,000 times lower than in the extracellular environment. This concentration gradient is utilised in fast responses to external stimuli, as intracellular concentration increases, leading to changes in muscle contraction, exocytosis, synaptic transmission, and gene expression [4].
ROS are highly reactive oxygen metabolites which can oxidise proteins, lipids and DNA. ROS are generated in normal cell metabolism such as the respiratory chain and the membrane-bound enzyme complex nicotinamide adenine dinucleotide phosphate (NADPH)-oxidases and contributes to cell signalling in physiological as well as pathological processes [5].
New treatment strategies are focusing on the application of physical stimuli for tissue regeneration. In this review we aim to examine electrical, mechanical and light stimuli as well as physical plasma application on cell signalling and behaviour with the focus on intracellular Ca2+ and ROS pathways.
Electric Field
Electrical signals play a crucial part in physiological processes, from brain activity to heart-beat. The electrophysiology of neurons and cardiomyocytes has been studied intensively as well as their response to external electrical stimuli. Deep brain stimulation and artificial cardiac pacemakers have long been the standard of care. However, it is not widely known that bone also responds to electrical signalling. Fukada and Yasuda first described the piezoelectric properties of dried bone [6], and piezoelectrical properties were also described in moisture bone later [7]. Through body movements, tissue deformation occurs in the bone, creating strain-related potentials. Also, bioelectrical potentials are generated by biological processes like aerobic glycolysis in the tissue. Especially fracture sites exhibit a negative voltage of 10–30 mV compared to undamaged tissue 1–2 cm away. In areas of negative polarisation, bone formation was detected [8]. Following these findings, many in vivo studies on the beneficial role of external electrical stimulation in bone healing have been conducted. A meta-analysis from 2011 evaluated 105 clinical studies and 35 in vitro studies [9]. The authors concluded that most studies favoured electrical stimulation, although no standard treatment is available, and the results are difficult to compare. This ambiguity is due to three possible modalities for bone growth stimulation: direct contact, capacitive coupling, and inductive coupling (pulsed electromagnetic field) [10]. Moreover, different parameters in the electric field magnitude, stimulation duration, current, voltage and frequency were used in these studies, making a comparison even more difficult [11]. For reproducible results, detailed information about electronic devices, the experimental set-up, and the applied stimulus parameters should be provided. This way, in silico simulations can estimate the electric field more accurately and make the assessment and interpretation of biological results easier [12].
In vitro experiments have shown a wide range of effects on cell functions. Improved cell-material interactions and proliferation were noticed after stimulation of osteoblasts on titanium surfaces [13] and anodized titanium [14, 15]. Pettersen et al. also described improved cell survival after pulsed electrical stimulation and enhanced collagen production [16]. Collagen I mRNA was found to be increased depending on the used voltage even when no enhanced proliferation was described [17]. Also alkaline phosphatase (ALP) activity and gene expression was found to be enhanced at low voltages indicating osteogenic differentiation. Furthermore, gene expression of bone morphogenetic protein 2 and osteopontin, which contribute to osteogenic differentiation, were elevated after direct current (DC) stimulation of pre-osteoblasts [18]. Capacitive coupled electrical stimulation with 0.7 V/mm and a high frequency pulse of 60 kHz was found to enhance osteogenic differentiation in pre-osteoblasts and mesenchymal stem cells in vitro [19].
Many studies report changes in Ca2+ concentration due to stimulation. Khatib et al. described 45 times higher intracellular Ca2+ concentrations in human osteoblasts 20 min after the beginning of DC stimulation with 2 V/cm. They found an influx of extracellular Ca2+ through stretch-activated cation channels (SACC) and a release from intracellular stores, as well as the activation of phospholipase C (PLC) to contribute to Ca2+ increase [20]. They hypothesised that the activation of voltage-gated calcium channels (VGCC) only occurs at higher field strengths. Bagne et al. found increased protein expression of VGCCs and calmodulin after direct stimulation, whereas only calmodulin was increased after electromagnetic treatment [21]. They proposed a Ca2+ influx either from extracellular or intracellular stores, leading to increased cytosolic Ca2+ concentrations and subsequent calmodulin activation. After binding Ca2+, calmodulin is able to bind specific proteins such as adenylate cyclase type-1, calmodulin kinases, and G-protein coupled receptors [22]. Calmodulin kinase I, II, and IV were shown to be expressed in osteoblasts and contribute to osteoblast differentiation through the activation of CREB/CRE signalling [23, 24].
Treatment with pulsed electromagnetic fields at 1 mT also led to increased proliferation, intracellular Ca2+ levels, and osteogenic differentiation in mesenchymal stem cells [25]. The authors also found greater protein expression of Wnt1 and β-catenin. They proposed an interdependent Wnt/Ca2+ and Wnt/β-catenin signalling pathway as increased intracellular Ca2+ enables β-catenin translocation into the nucleus. The canonical Wnt pathway is well known to play an essential role in mesenchymal stem cell differentiation and osteoblast maturation [26]. Other studies reported the activation of the mTOR (mammalian Target of Rapamycin) pathway after pulsed electromagnetic field treatment increased proliferation [27]. The activation of mTOR depends on the phosphatidylinositol 3-kinase (PI3K) activity [28]. PI3K activity is enhanced upon calmodulin binding and therefore sensitive to Ca2+ dynamics [29]. The mTOR complexes facilitate bone formation and osteoblastic differentiation [30]. Also, some Wnt ligands were shown to exhibit their beneficial effects on osteoblast proliferation and activity via the activation of mTOR signalling [31].
Changes in the Ca2+ oscillation patterns occur during osteogenic differentiation of mesenchymal stem cells. Both intensity and amplitude of Ca2+ spikes were decreased in mature osteoblasts compared to the stem cells. During treatment of mesenchymal stem cells with osteoinductive factors or direct contact electrical stimulation, Ca2+ spike patterns were altered and resembled osteoblast-specific patterns. Induction of osteogenic differentiation was confirmed by increased ALP levels and extracellular Ca2+ deposits [32, 33]. Sun et al. discussed membrane depolarisation to occur after stimulation with high voltages. In contrast, Bhavsar et al. treated adipose-derived mesenchymal stem cells with smaller voltage DC and observed hyperpolarisation during electrically enhanced osteogenic differentiation [34].
Another effect of electrical stimulation is observed during DC stimulation. Cells orientate themselves perpendicular to the electric field and migrate in the direction of the electrodes [35, 36]. While adipose-derived mesenchymal stem cells were found to migrate towards the anode [37], osteoblast cell lines migrated towards the anode or cathode [38].
Rohde et al. described directed migration towards the anode and increased migration speed under DC stimulation in primary human osteoblasts [39]. However, this effect was not abolished by inhibiting the transmembrane Ca2+ flux. Despite these results, the inhibition of VGCCs by nifedipine during DC stimulation of adipose-derived mesenchymal stem cells abolished the previously seen beneficial effect on migration and calcium deposition. The blockage of voltage-gated sodium and potassium channels also inhibited enhanced migration [40]. In contrast to Rohde et al., Özkucur et al. found the migration of osteoblasts as well as redistribution of focal adhesions and the actin cytoskeleton to be dependent on Ca2+ in the cell culture medium and possibly Ca2+ influx through VGCCs. However, they were applying unphysiologically high electric field strengths of 14 V/cm compared to field strengths of 0.1–0.03 V/cm in fracture sites [38].
In consistency with the cell orientation, the cytoskeleton was observed to orientate perpendicular to the DC field as well [41]. While the mesenchymal stem cells at 0.1 V/cm field strength appeared to have denser F-actin structures near the cathode, Titushkin and Cho described an impairment of actin filaments after stimulation with 2 V/cm [42].
Apart from Ca2+ signalling, many other cellular mechanisms are discussed to contribute to beneficial or adverse effects of electrical stimulation. For instance, the direct contact stimulation leads to the generation of faradic by-products such as H2O2 in the cell culture medium. Stimulated medium alone was able to influence cell viability and gene expression of osteoblasts [18]. Leppik et al. also discussed the formation of lipid rafts and ATP synthesis to trigger intracellular signalling after electrical stimulation [10]. Lipid rafts were found to cluster during electrical stimulation, leading to the activation of intracellular signalling molecules such as PI3K [43].
Taken together, intracellular Ca2+ signalling plays a major part in mediating the effects of electrical stimulation of bone cells. The origin of the Ca2+ signal may vary depending on the cell type or stimulation set up and parameters. Since there is no standard treatment for electrical stimulation in bone healing, applied parameters and the results are often difficult to compare. Identified pathways include the activation of calmodulin, mTOR, and Wnt signalling, which affect cell proliferation, differentiation, and migration in vitro (Fig. 1). Understanding the molecular mechanisms of electrical stimulation is essential to optimise stimulation parameters in bone healing treatments and further improve patients’ lives.
Possible mechanisms of electrical stimulation induced proliferation and differentiation in mesenchymal stem cells and pre-osteoblasts. Electric field application leads to an increase of intracellular Ca2+ and subsequent activation of gene expression. CREB/CRE cAMP response element-binding protein/cAMP response element; ER endoplasmic reticulum; IP3 inositol-triphosphate; mTOR mammalian target of rapamycin; NF-AT nuclear factor of activated T-cells; PI3K phosphatidylinositol 3-kinase; PIP2 phosphatidylinositol-4,5-bisphosphate; PIP3 phosphatidylinositol-3,4,5-trisphosphate; PLC phospholipase C; SACC stretch-activated cation channel; VGCC voltage-gated calcium channel. (Parts of the figure were drawn by using pictures from Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License, https://creativecommons.org/licenses/by/3.0/)
Mechanical Forces
The whole human organism and its physiological processes are stimulated by mechanical forces, e.g., by the general earth gravitation (9.807 m/s2) or by shear stress due to blood flow and pressure in the vascular system. It is well known from long-term space flights that gravitation has a remarkable impact on muscle and bone mass. Mechanical loading exercises can regenerate bone mass and function, and are also required to maintain bone health whilst aging [44]. The weightlessness during human space flights led to bone loss of 1.5% per month in the femoral neck of the astronauts and cosmonauts, which was detected by dual-energy X-ray absorptiometry [45].
In tissues, the cells are permanently influenced by dynamic mechanical stimuli in the environment such as shear forces, osmotic stress, and stretch via the extracellular matrix molecules and their nano-topographical cues [46]. In in vitro experiments, the mechanical impact on cells was clearly shown. Microgravity conditions (absence of gravitational and, therefore, load-bearing forces) caused a decrease in osteoblast cellular integrity accompanied by smaller and fewer focal adhesions, thinner cortical actin, and stress fibres [47]. Shear stress via pulsating fluid flow influenced the live-cell volume of MC3T3 osteoblasts, which was decreased by half. At the same time the protein expression of intracellular phospho-paxillin and the receptor subtype integrin-α5 was increased more than twofold [48]. In another approach, mechanical stress via an orbital shaker induced signal transduction in erythroblasts, i.e., the signalling molecule extracellular-signal related kinase (ERK), was enhanced in a Ca2+ dependent process where mechanosensitive channels were involved [49].
Cells are able to sense mechanical forces from the surrounding tissue and neighbouring cells. Specific mechanosensitive ion channels are located in the cell membrane, and their components Piezo1 and Piezo2 [50,51,52] were recently structurally identified by electron cryo-microscopy by Ardem Patapoutian’s work (Nobel Prize in 2021). The multipass transmembrane piezo protein Piezo1 is large with 300 kDa [51, 52]. Notably, several groups suggest that the response of Piezo1 to mechanical forces from outside the cell is dependent on the cytoskeleton’s integrity and scaffold proteins [53, 54]. Atomic force microscopy (AFM)-based mechanical stimulation of Piezo1 in living cells showed that Piezo1 channels were sensitized to mechanical pulling when collagen IV, which is responsible for the mechanical connection in the basal lamina, was the mediator between the cell membrane and the cantilever of the AFM [54]. However, Piezo1 activation was lower for ECM-coated AFM pulling than for AFM pushing with 33 nN and 200 nN, respectively [46, 54].
Besides these mechanosensitive cationic ion channels, two distinct mechanical sensor points in the cell membrane were recovered—the integrins within focal adhesions connected to the extracellular matrix (ECM) and the cadherins in cell–cell contacts. In experimental approaches, forces in the nm-dimension were found between cell–cell contact sides in epithelial cells with approximately 100 nN and between cells and the ECM with 200–400 nN [55]. The transmembrane adhesion receptors—the integrins—are connected to the actin cytoskeleton in the cell via adaptor proteins (e. g. paxillin, vinculin), building a “bridge” from outside the cell to its interior [56,57,58]. Thus, physical forces can be transmitted outside-in, called mechanotransduction [59], which means the conversion of mechanical signals into biochemical signals [60]. As a result, intracellular signalling molecules can be activated by phosphorylation, e. g. p-ERK and p-Akt [61, 62] or released from intracellular stores, such as the calcium ions (iCa2+) as “second messengers” [63, 64]. A specific integrin α5 receptor has immense importance for anabolic actions on skeletal tissue after mechanical loading. In a knock-out mouse model the α5 deficiency lead to, i.a., impeded load-induced connexin Cx43 hemichannel opening, resulting in inhibited endosteal bone formation [65]. Integrin-mediated signalling is specific since mechanically stressing of a non-integrin receptor (transferrin) did not provoke the iCa2+ rise in U2-OS osteoblastic cells [66]. Cyclic forces of 1 Hz via paramagnetic beads bound to β1- and α2-integrin receptors were more effective than continuous stress in a magnetic drag force device [62]. The induced biochemical signals are fundamental for the cell physiological processes proliferation, differentiation, and migration. Other authors identified the same downstream signalling pathway and showed that mechanically stretching the whole adherent cells (10 min cyclic mode) induced a clear phosphorylation of ERK1/2 but not of Akt [67]. In their experiments cells grew on fibronectin, a ligand for α5β1integrins. Interestingly, there is an interrelation to the ROS signalling in cells during the attachment phase since integrin-mediated cell adhesion and growth induced ROS sources, e.g. the mitochondria [67, 68].
The actin cytoskeleton plays an important role in mechanotransduction. In a work by Wang et al. [69], the mechanical role of these microfilaments was elucidated in 6 h-adherent endothelial cells. The cytoskeletal disrupter cytochalasin D inhibited cell stiffness by 50%. Under mechanical exposition, it was found that integrins directly stably link to the actin cytoskeleton. In human leukocytes, the β2-integrins were cross-linked with antibodies, and after Triton X-100 extraction, this induction of a physical linkage of the integrin subtype to actin was visible [70]. This physical interaction seems to be essential in integrin-mediated signal transduction [71].
The cell–cell contact sides called cadherins also transduce and respond to mechanical forces and interplay also with the integrins via the actin-myosin network [60] (Fig. 2). The coordinated interaction between integrins and cadherins mechanically connects the actin cytoskeleton to neighbouring cells and to the ECM in order to regulate multicellular processes on the tissue level, such as migration and patterning during morphogenesis [60].
Transmembrane integrins and cadherins—responsible for mechanically sensitive cell connections. Integrin-based focal adhesions to the extracellular matrix (A) and cadherin-based adherens junctions in cell–cell contacts (B) transmit mechanical signals via the actin–myosin network (C). (Image from Mui, Chen and Assoian [60], adapted with permission from J Cell Sci, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4813297/bin/joces-129-183699-g1.jpg)
Vinculin as one of the essential adaptor proteins in focal adhesions, connecting talin and actin [72] (Fig. 3), was found to be also localised in cadherin-dependent adherens junctions. Under high intracellular tension, the α-catenin in these junctions exposes a binding site for vinculin. Thus vinculin links adherens junction to F-actin [73], thereby forming a mechanically driven crosstalk between cadherins and integrins [60]. These interactions between the cadherin-mediated cell–cell and the integrin-mediated cell–matrix adhesions can be cooperative and interdependent to achieve tensional homeostasis in cells [60] and tissue. Thus, trans-cellular force transmission to neighbouring cells occurs not via integrin-type cell–matrix adhesions but via cadherin-type cell–cell adhesions [74].
Complex mechanical anchorage of cells in their micro-environment with cell–cell and cell—extracellular matrix adhesions. A Osteoblastic cells are strongly attached to each other and to the basal surface. The intracellular actin cytoskeleton shines through with its relief-like stress fibres bridging intracellularly both cell contact types, thus providing a mechanically stabile scaffold. (24 h cell growth on glass coated with plasma polymer [77], gold sputtered; SEM DSM 960A, Carl Zeiss, scale bar 25 µm, image from HR). B HCMEC cells connected via cadherin contacts (green). The actin cytoskeleton (red) is not only organized in stress fibres but also submembranously distributed nearby the cell–cell borders (nucleus in blue, LSM780, Carl Zeiss, scale bar 10 µm, image from P. Müller, Dept. of Cell Biology, Rostock). C Adaptor protein vinculin (red) co-localised with actin cytoskeleton fibre tips in focal contacts of SLUlar1 cell–matrix adhesions (LSM780, Carl Zeiss, scale bar 5 µm, image from HR)
The focal adhesion kinase (FAK) is also a central adhesion protein essential for cell mechanical stability in the cells’ focal adhesions. The Fabry group discovered that FAK-deficient (-/-) mouse embryonic fibroblasts showed lower cell stiffness, reduced cell areas, and reduced adhesion strength [75]. They also observed that the increased cytoskeletal dynamics was attended by reduced stability of the cytoskeleton compared to the wild-type cells.
To receive all the detailed information about cell physiological processes, cells can be mechanically stimulated in vitro by several devices such as compressive tensile stresses, flow-induced shear stress, cell stretching devices, microfluidics [47, 48, 76], microgravity [47] or via magnetic drag force devices [62, 63].
Physical Plasma
Physical plasma refers to the fourth state of matter, defined as a partially ionised gas [78]. Physical plasma is generated by the input of energy, e.g., heat energy, to a neutral gas. Plasma consists of charged particles (electrons, ions), neutral and electrically excited atoms and molecules, UV photons, and radicals. In addition to the industrial application of plasma in metal processing and automotive engineering, its therapeutic benefits in medicine are also being increasingly researched. Thermal argon plasma can be used, for example, for heat cauterisation during endoscopic surgery or sterilisation of medical devices [79].
Especially for medical applications, plasma with lower temperatures produced under atmospheric pressure is more accessible and versatile. It can also be applied to living tissue, which resulted in the rise of a new field of research called plasma medicine. To produce cold atmospheric pressure plasma (CAP), the carrier gas is usually exposed to an electric field. Neutral gas always contains a small proportion of electrons and ions. These are accelerated by the electric field and generate new electrons and ions in collisions with neutral atoms and molecules or with the electrode surfaces [80]. The plasma’s density, temperature, and composition can be influenced by changing the electric field (e.g., field strength, pulse duration) [81, 82]. Due to the variety of atmospheric plasmas generated (source: Jet or DBD; gas: Ar or He ± O2) and energies applied to generate a plasma (discharge voltage 1–6 kV and frequency of 2.5 kHz for kINPen®MED (neoplas tools GmbH); discharge voltage 14 kV and frequency of 100–400 Hz for PlasmaDerm® (Cinogy GmbH) [83, 84] there are multiple parameters influencing the plasma efficacy [84]. This is why, at the moment, a standardisation of the term “plasma dose” is being discussed worldwide, although a final decision has not yet been achieved. In a complex experimental approach with 18 varying parameters and subsequent modelling, it was shown that especially the parameters “treatment time”, “cell number” and “volume of fluid” have an influence on the anti-tumor effect of CAP, while e.g. the distance between plasma jet and medium is less relevant [85]. In comparative studies, it is therefore enormously important to select uniform conditions for the cultivation of the cells [86, 87]. Therefore, one of the main tasks for the future is the international standardisation of CAP sources for medical use both in terms of their technical parameters (beyond the general regulations for medical devices) and their biological performance. This will be crucial for the comparability and reproducibility of research efforts.
Generally, there is a consensus that the amount and the mixing ratio of the generated reactive species are crucial for the plasma effect. Lately more insight was gained into the dependence of reactive oxygen and nitrogen species (RONS) composition, plasma density and electron temperatures on target characteristics and plasma-target interactions [88].
The effects of CAP have been described in numerous studies; for example, it has a disinfecting effect (bacteria, fungi, viruses), promotes tissue regeneration (pH modulation, angiogenesis), is anti-inflammatory and anti-carcinogenic (pro-apoptotic) [89,90,91]. The treatments of chronic wounds with anti-inflammatory, disinfecting effects and promoted wound healing have also been successfully tested clinically [91, 92].
In recent years, the potential use of CAP in cancer therapy has been investigated. The controlled application of CAP can induce apoptosis of cells in a defined area. Thus, eliminating malignant lesions without significant necrosis and subsequent inflammation becomes possible. Numerous publications have demonstrated CAP-mediated inhibition of cell invasion and migration [93, 94] and apoptosis induction [94,95,96,97] in various cancer cell lines. More recently, the field of plasma immunology has been explored as new evidence suggests the involvement of the immune system in the anti-tumorigenic effects of CAP in vivo [98]. Compared to conventional cancer therapies, CAP treatment has been shown to be highly selective towards cancer cells [94, 96, 99]. This observation places CAP as a promising approach in tumour therapy. Therefore, it is essential to explore the mechanisms behind the potential selective effect of CAP.
It has not yet been adequately and comprehensively clarified by what means CAP unfolds its anti-carcinogenic, anti-inflammatory and regenerating efficacy. It is well accepted that RONS play a major role. In this context, not only the reactive species in the plasma itself are meant, but also RONS that are formed intracellularly by plasma treatment [89, 100]. Cells produce a well-regulated amount of RONS due to normal metabolism, and it is thus tolerated by the cells. However, according to the concept of oxidative eustress and distress, lethal effects such as apoptosis may occur if a maximum tolerable threshold is exceeded. Therefore, depending on the amount of RONS present in the tissue, a proliferation-promoting or lethal effect can be generated by CAP treatment (Fig. 4). Low concentrations of, e.g., H2O2 or peroxynitrite can promote a protective reaction by increasing the amount of reducing equivalents, such as NADPH [101]. In addition, the induction of the hypoxia-inducible factor 1α (HIF1α) may lead to increased glycolysis and pro-angiogenic effects [102].
Intracellularly, a high influx of RONS or impairment of RONS scavenging pathways leads to RONS accumulation resulting in organelle and cell dysfunction. The major RONS buffer systems in mitochondria are glutaredoxin, glutathione, and thioredoxin systems. The conversion of O2− to H2O2 is performed by the SOD (superoxide dismutase) protein family. In the mitochondrial matrix, dismutation is mainly by SOD2 (MnSOD; manganese-dependent superoxide dismutase), whereas in the intracellular space, dismutation is by SOD1 (Cu, Zn-SOD). Decomposition of H2O2 into O2 and H2O then occurs via the glutathione redox system, which includes glutathione reductases, peroxidases, and peroxiredoxins [103].
If the RONS level exceeds the cellular detoxification capacity, RONS lead to the activation of several signalling pathways. The excessive intracellular formation of RONS, for example, leads to DNA damage [89]. Reactive species also trigger lipid peroxidation reactions, which can lead to damage in the cell membrane and thus to an increased influx of reactive species into the cell, where they influence the metabolism of the cells via the Ca2+ signalling pathway, activation of NF-κB, or trigger apoptosis through oxidative stress.
ROS such as O2−, HO·, and H2O2 can influence the intracellular Ca2+ balance. Mild oxidative stress transiently increases the Ca2+ concentration via release from intracellular stores. In contrast, severe oxidative stress causes an influx of Ca2+ from the extracellular space [104]. Studies on melanoma cells showed a marked increase in intracellular Ca2+ after CAP treatment. This increase was due to Ca2+ release from intracellular stores (mitochondria and endoplasmic reticulum). A fundamental aspect within the interaction between intracellular Ca2+ and ROS is their tightly interconnected regulation. Since Ca2+ signalling is crucial for the production of ROS, and vice versa, intracellular Ca2+ signalling can be controlled by ROS [105, 106]. The combination of CAP with other treatment regimens may exploit this effect, as calcium homeostasis influences the cellular reprogramming of cancer cells. Recently, the combination of CAP with hyperthermia, a heat treatment at 42 °C, was described, demonstrating the activation of transient receptor potential melastatin 2 (TRPM2) and consequently an increased intracellular calcium level [107].
A unique mechanism is the chemical reaction of RONS with the sulfhydryl groups of redox-sensitive proteins, resulting in a covalent and reversible modification of the highly reactive cysteine residues in these proteins. Redox-sensitive proteins with regulatory functions including ion translocators, DNA topoisomerases, and signalling proteins (protein phosphatases and kinases, nuclear factor kappa B (NF-kB), p53, and G proteins) have reactive cysteines at the active sites, DNA-interacting motifs, and signalling protein interfaces. RONS cause stepwise oxidation of cysteines to sulfonic acids. The oxidized cysteines lead to irreversible loss of biological activity in most proteins. Experimental data showed that several genes, including members of redox-sensitive proteins such as NF-kB, B-cell lymphoma 2 (Bcl-2), and tumor necrosis factor family, were strongly affected by CAP [108]. Especially the Bcl-2/Bax (Bcl-2-associated X protein) ratio is crucial for the induction of apoptosis via the mitochondria-mediated pathway. CAP treatment has been shown to increase pro-apoptotic Bax protein while decreasing anti-apoptotic Bcl-2 [109].
The modulation of ROS levels or the targeted use of antioxidants for cancer treatment is not new. A significant increase in ROS levels, for example, is also the basis for the anti-tumorigenic effect of chemotherapeutic agents such as cisplatin, carboplatin, and doxorubicin [110].
The influx of ROS into the cell could take place through membrane-penetrating aquaporins. It is known that H2O2 can enter the cytoplasm via AQP1 or AQP3. This is relevant since it is argued that several cancer cells express a higher number of aquaporins than their non-cancerous counterparts [111]. However, new work suggests that the mere expression level of aquaporins does not allow a correlation with the sensitivity to plasma treatment [112]. It is possible that the activity of the aquaporin channels also plays a significant role. Other mechanisms that could explain the selective effect of plasma are considered to be the different levels of detoxifying enzymes, such as glutathione peroxidase, catalase, or peroxiredoxin [113, 114]. Different cholesterol content in the membrane is also discussed as a causal factor since a low cholesterol content can favour pore formation [115].
In addition to direct treatment with CAP, indirect treatment with plasma-activated fluids is also possible, which shows comparable efficacy [89, 116]. In plasma-activated fluids, long-lived reactive species such as H2O2 and NO2− are formed [98]. The efficacy of plasma-activated cell culture medium (PAM) is attributed to long-lived, stable organic peroxides formed by the reaction of amino acids or proteins with short-lived reactive species [89]. Peroxidised amino acids can react by polymerisation to form larger compounds. Due to the long-lived reactive species, argon plasma-activated medium continues to produce effects for up to 21 days after the medium has been treated [117]. Stable plasma-activated fluids offer further clinical application possibilities besides the direct application of CAP.
Future challenges include standardisation of plasma sources and treatment doses for biological and medical applications, as this is one of the most important issues in plasma medicine [118]. Prospective applications favour the combination of plasma with other treatments, such as nanoparticles, where the uptake of nanoparticles is enhanced by the joint action of RONS and electric fields [119], with the complexity of the plasma being a key contributor.
Light
Light energy has many effects on human physiology and health. Some of these mainly beneficial effects, such as signal transmission via the light-sensitive cells of the eye or vitamin D production by skin cells, are well described. Nevertheless, there are also negative, more unspecific effects such as damage to the skin by ultraviolet (UV) radiation. Light is an electromagnetic wave that transports energy through space. For example, light energy from the sun is transported into our atmosphere and has an influence on the environment here (Fig. 5). Light energy is not continuous, but arrives in discrete units called photons. Photons are the particles of light that are electrically neutral and massless. The energy of photons is inversely proportional to the wavelength of the electromagnetic wave. That is, the shorter the wavelength, the more energetic the photon; the longer the wavelength, the less energetic the photon. When photons strike matter (e.g., the human body), they can be absorbed by its atoms and transfer their energy via electrons from the ground state to an excited state and vice versa (Fig. 5).
Simplified depiction of the electromagnetic waves emitted by a light source, which act on the environment in the form of photons (also on the cells of the human body). These photons then strike atoms, by which they are absorbed and transfer this energy to electrons, which shift from the ground state to an excited state and vice versa (images created with pictures from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License)
The energy of light can be used for a variety of medical therapies. When light in the visible and near-infrared spectral range are used to treat diseased or damaged tissue without emitting thermal energy, it is defined as photobiomodulation therapy (also called photomodulation therapy or, when light is applied with a laser, low-level laser therapy/LLLT). This form of therapy represents a mostly non-invasive, low-threshold therapeutic approach in regenerative medicine. In photobiomodulation, the light-specific electromagnetic force is used to trigger chemical and/or physical processes in the treated tissue at the molecular level, which modulates the biological activity of the tissue. Photobiomodulation therapy has been clinically applied or studied to treat a wide variety of medical disorders, including the promotion of tissue repair and restoration and stimulation of chronic wound healing [120].
Although photobiomodulation therapies have been known for many decades and there is ample evidence of therapeutic efficacy, scientific validation of this topic is still quite limited. The reason for this comparatively weak scientific research base can be attributed to several aspects: (a) the complexity of the many different light parameters (e.g., wavelength, energy, irradiation time, and treatment intervals), (b) the large heterogeneity of the diseases treated, and (c) the poorly understood molecular mechanisms at the cellular or tissue level. The joint presence of the above-mentioned three aspects makes the subject considerably confusing and thus complicates the creation of a uniform data basis.
Presumed photoacceptors relevant for photobiomodulation: Photochemical reactions depend on the absorption of light by a system (Grotthuss–Draper law). The molecules mediating the biological response to light are photoacceptors (chromophores). The energy retained in the photoacceptor after light activation can initiate a photochemical reaction in the cells. Different cellular photoacceptors exist, and their activation is dependent on the wavelength, among others [121, 122]. One comparatively well-studied potential photoacceptors is cytochrome C oxidase (CCO). This is the terminal enzyme of the mitochondrial electron transport chain. CCO contains two copper centres and two hemes which are involved in redox reactions within the enzyme. There is some evidence that CCO functions as a photoacceptor and transducer of photosignals in the red and near-infrared regions of the light spectrum [123, 124]. However, the comprehensive theory for the photochemical response in mitochondria is currently under development, as recent studies have shown that the isolated CCO enzyme has not demonstrated significant effects on light exposure. Thus, the exact processes that occur in the CCO enzyme and the mitochondria during absorption of light energy have not yet been elucidated [125,126,127]. However, regardless of the as-yet unclear mechanisms of CCO activation, red and near-infrared light increases mitochondrial membrane potential, leading to an increase in adenosine triphosphate (ATP) synthesis and changes in ROS and nitric oxide (NO) concentrations [128]. In addition, there is evidence of light-induced changes in mitochondrial ultrastructure [129] and alteration of inflammatory activities of the ‘nuclear factor kappa-light-chain-enhancer of activated B cells’ (NF-κB) [130]. However, the exact effects of such light exposure are difficult to predict and clearly depend on the dose, the cell type studied, and the specific experimental conditions.
Another group of potential photoacceptor molecules is the opsins. Opsins have only recently begun to be studied for their function beyond vision, as they have only been discovered in recent years in various cell types outside the eye (e.g., skin, brain, adipose tissue, blood vessels) [131,132,133,134]. Opsins are G protein-coupled receptors that can excited by blue or green light [135]. Several publications have investigated the role of opsins in blue light-mediated signalling both in vitro and in vivo. Barreto Ortiz et al. found opsins in arterial smooth muscle cells where they are involved in the regulation of vasorelaxation [131]. In addition, the presence of opsins is shown in human adipose tissue and subcutaneous white adipocytes [134]. However, the molecular and cellular mechanisms of the photobiomodulated signalling pathway have not been fully elucidated [136]. It is suggested that a downstream target of opsins are the ligand-gated ‘transient receptor potential’ (TRP) channels [122, 137]. At least 28 TRP cation channel superfamily members were discovered in mammals, which can be subdivided into two groups and six families (among them are TRP channels, which possess Ca2+-selectivity). At present, there is currently limited evidence that light-mediated activation of TRP channels is responsible for some of the mechanisms of action of photobiomodulation. TRP are redox sensors activated by hydrogen peroxide (H2O2) and nitric oxide (NO), for example, and their activation alters gene transcription [138].
Flavins or flavoproteins are another group of tentative photoacceptors in photobiomodulation. These photoacceptors show excitability by light in the blue wavelength range [139]. Flavoproteins are found in the electron transport chain, and blue light is thought to lead to the reduction of oxygen (O2) to superoxide (O2−), increasing ROS levels and affecting mitochondrial activity, as is the case with red and near-infrared light [140].
In addition, there is some evidence that light exposure in cell-free systems can also affect biologically important molecules beyond those previously described in cells. For example, it has been reported that the latent form of transforming growth factor β (TGFβ) can be activated by light exposure [141]. Moreover, exposure of an ATP solution to red and near-infrared light resulted in changes in ATP reactivity and Mg2+ binding capacity [142].
Wavelength-dependent penetration depth into tissues: Most of the effects of photobiomodulation have been described for the treatment with red to near-infrared wavelength ranges. This is probably due to the corresponding chromophores and the different penetration depths into the treated tissue. The penetration depth is much less for blue light than for red or near-infrared light. While blue light has a penetration depth of about 1 mm, near-infrared light reaches a penetration depth of up to 5 mm [143, 144]. Therefore, the clinical application of blue and green light might be limited due to the lack of tissue penetration [122].
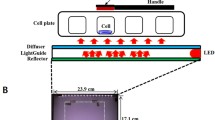
Effects of light exposure on mesenchymal stem/stromal cells: As mentioned above, light exposure has been shown in numerous studies to affect cell proliferation, cell metabolism, and the inflammatory response, and thus can fundamentally support tissue healing. Because of its role in regeneration and its use in regenerative therapies, mesenchymal stem/stromal cells (MSC) are one central area of interest in studies of the effects of light energy [145]. MSC can self-renew, differentiate into various specialized cell types, and have immunomodulatory properties. MSC have been detected in almost all tissues of the body, including comparatively easily obtainable tissues such as bone marrow, adipose tissue, umbilical cord, and placenta [146]. With these described properties, MSC have the potential to support the regenerative capacity of tissues, and this potential has been shown to be influenced by light exposure. In particular, the exposure to the red and near-infrared wavelength range showed apparent effects on MSC of different origins: For example, Tani et al. were able to achieve a higher degree of osteogenic differentiation after treatment of human bone marrow MSC with light in the red and infrared wavelength range [147]. Also, human umbilical cord MSC were proven to have a higher osteogenic differentiation capacity when treated with red light [148]. In addition, the treatment of umbilical cord MSC with red and near-infrared light could improve the efficiency of neural differentiation under specific differentiation stimuli [149]. This is only a small selection of the effects already described, but all of these effects depended on the wavelengths used. Our experiments in an ongoing study on the effects of different light wavelengths on adipose tissue-derived MSC (adMSC) also showed significant differences depending on the wavelengths used in the analysis of the “in vitro wound healing capacity” using the so-called scratch assay (Fig. 6, unpublished results). The treatment with the blue light of an adMSC suspension in a light exposure chamber at a wavelength of 455 nm resulted in an energy-dependent increase in “in vitro-wound healing potential”: adMSC treated with blue light for 7.5 min (energy of 414 J in the light exposure chamber) almost completely overgrew the surface, whereas untreated cells still showed a clear gap. Shorter treatment with blue light (2.5 min) and thus a lower energy input (energy of 138 J) resulted in a state between the control and 7.5 min treatment. Whether these differences are solely due to the increased migratory activity of adMSC caused by blue light exposure and what molecular mechanisms are involved is part of the currently ongoing study. The exposure of adMSC to the red light of wavelength 660 nm and a similar power input showed no effect in this experimental setup (data not shown).
Scratch assay of adipose tissue-derived mesenchymal stem cells under A control conditions (not light-exposed), and 2 days after application of blue light (455 nm), B for 2.5 min and C for 7.5 min. [LED light source, power input 921 mW; cell staining with calcein-AM (AAT Bioquest) 15 h after scratching; fluorescence microscope WiScan® Hermes (Idea Bio-Medical)]
The finding that light exposure affects the migratory ability of MSC has been rarely investigated. Ong et al. were also able to demonstrate an increased migratory capacity in human MSC. However, the experimental approach was completely different from our experiments, as a transwell chamber system was used, and the cells were irradiated with a green LED light source (wavelength 530 nm) at a distance of 30 cm to the transwell chamber with different power densities (11.3, 22.8 and 66.4 mW/cm2). Due to the different experimental dimensions (cells in suspension exposed in an integrating sphere vs. adherent cells in a transwell chamber with single-sided light exposure), a direct comparison of light energy delivery is not feasible. In the transwell experiments from Ong et al. the cells migrated away from the green light source, whereas orange light exposure (wavelength 625 nm) did not affect the migration activity of MSC [150].
In summary, the experiments performed with MSC resulted in changes in proliferation, differentiation, and other regeneration-promoting properties such as migratory capacity or reduction of inflammation. Therefore, it can be assumed that photobiomodulation therapy can also influence tissue regeneration via direct light-induced effects in MSC [145]. However, there are explicit dependencies on the wavelength used, the energy input, the origin of the MSC, and other experimental characteristics.
Despite the great progress in the study of the effects and molecular mechanisms of light-induced effects in recent years, many technical and biological aspects that may affect the safety of photobiomodulation therapy are still open. Basically, the available data are often difficult to compare due to different experimental approaches using different cell culture methods, light exposure techniques, and different light sources. Therefore, further efforts are needed to better understand both the technical basis and the cellular mechanisms underlying the effects of photobiomodulation.
Conclusion
Electrical fields, mechanical forces, physical plasma and light are applied in a wide range of research areas with the common focus on the development of regenerative therapies. Medical devices, which apply these stimuli to tissues or cells in cultures, should run on optimised parameters, as even small changes in the applied forces lead to changed cellular outcomes. For example, electrical stimulation was described to increase or decrease apoptosis and cell proliferation depending on the used voltage, treatment time, cell type, and stimulation set up [151]. Although we described the application of different physical factors on various cell types, a common cell signalling pattern emerges. We found the increase of intracellular Ca2+ and ROS concentrations to be important for cell signalling and downstream gene expression. ROS generation was described to contribute to aging, cancer and neurodegenerative diseases, but in the last decades ROS has been discovered to contribute to physiological cell signalling as well [152]. Also ROS and Ca2+ signalling were found to be connected and their cross-talk taking place not only in physiological but also in pathological processes [105, 153].
Therefore, our review outlined important mechanisms in proliferation, differentiation and wound healing due to stimulation with physical factors.
References
P.A. Janmey, D.A. Fletcher, C.A. Reinhart-King, Stiffness sensing by cells. Physiol. Rev. (2020). https://doi.org/10.1152/physrev.00013.2019
M. Witkowska-Zimny, K. Walenko, A.E. Wałkiewicz, Z. Pojda, J. Przybylski, M. Lewandowska-Szumieł, Effect of substrate stiffness on differentiation of umbilical cord stem cells. Acta Biochim. Pol. 59, 261–264 (2012)
J.H. Wen, L.G. Vincent, A. Fuhrmann, Y.S. Choi, K.C. Hribar, H. Taylor-Weiner, S. Chen, A.J. Engler, Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. (2014). https://doi.org/10.1038/nmat4051
M.S. Islam (ed.), Calcium signalling, in Springer eBooks Biomedical and Life Sciences, vol. 1131, 2nd edn. (Springer International Publishing, Cham, 2020)
A.V. Snezhkina, A.V. Kudryavtseva, O.L. Kardymon, M.V. Savvateeva, N.V. Melnikova, G.S. Krasnov, A.A. Dmitriev, ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell. Longev. (2019). https://doi.org/10.1155/2019/6175804
E. Fukada, I. Yasuda, On the piezoelectric effect of bone. J. Phys. Soc. Jpn. (1957). https://doi.org/10.1143/JPSJ.12.1158
A.S. Nowick, Piezoelectric properties of bone as functions of moisture content. Nature (1975). https://doi.org/10.1038/253626a0
J. Black, Electrical Stimulation: Its Role in Growth, Repair and Remodeling of the Musculoskeletal System (Greenwood Press, Westport, 1986)
M. Griffin, A. Bayat, Electrical stimulation in bone healing: critical analysis by evaluating levels of evidence. Eplasty 11, e34 (2011)
L. Leppik, K.M.C. Oliveira, M.B. Bhavsar, J.H. Barker, Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. (2020). https://doi.org/10.1007/s00068-020-01324-1
E. Pettersen, J. Anderson, M. Ortiz-Catalan, Electrical stimulation to promote osseointegration of bone anchoring implants: a topical review. J. Neuroeng. Rehabil. (2022). https://doi.org/10.1186/s12984-022-01005-7
K. Budde, J. Zimmermann, E. Neuhaus, M. Schroder, A.M. Uhrmacher, U. van Rienen, Requirements for documenting electrical cell stimulation experiments for replicability and numerical modeling∗, in Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference (2019). https://doi.org/10.1109/EMBC.2019.8856863
S. Bodhak, S. Bose, W.C. Kinsel, A. Bandyopadhyay, Investigation of in vitro bone cell adhesion and proliferation on Ti using direct current stimulation. Mater. Sci. Eng. C (2012). https://doi.org/10.1016/j.msec.2012.05.032
B. Ercan, T.J. Webster, Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int. J. Nanomed. (2008). https://doi.org/10.2147/ijn.s3780
B. Ercan, T.J. Webster, The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces. Biomaterials (2010). https://doi.org/10.1016/j.biomaterials.2010.01.078
E. Pettersen, F.A. Shah, M. Ortiz-Catalan, Enhancing osteoblast survival through pulsed electrical stimulation and implications for osseointegration. Sci. Rep. (2021). https://doi.org/10.1038/s41598-021-01901-3
F. Sahm, J. Ziebart, A. Jonitz-Heincke, D. Hansmann, T. Dauben, R. Bader, Alternating electric fields modify the function of human osteoblasts growing on and in the surroundings of titanium electrodes. Int. J. Mol. Sci. (2020). https://doi.org/10.3390/ijms21186944
K. Srirussamee, S. Mobini, N.J. Cassidy, S.H. Cartmell, Direct electrical stimulation enhances osteogenesis by inducing Bmp2 and Spp1 expressions from macrophages and preosteoblasts. Biotechnol. Bioeng. (2019). https://doi.org/10.1002/bit.27142
B.M. de Sousa, C.R. Correia, J.A.F. Ferreira, J.F. Mano, E.P. Furlani, M.P. Soares Dos Santos, S.I. Vieira, Capacitive interdigitated system of high osteoinductive/conductive performance for personalized acting-sensing implants. npj Regener. Med. (2021). https://doi.org/10.1038/s41536-021-00184-6
L. Khatib, D.E. Golan, M. Cho, Physiologic electrical stimulation provokes intracellular calcium increase mediated by phospholipase C activation in human osteoblasts. FASEB J. (2004). https://doi.org/10.1096/fj.04-1814fje
L. Bagne, M.A. Oliveira, A.T. Pereira, G.F. Caetano, C.A. Oliveira, A.A. Aro, G.B. Chiarotto, G.M.T. Santos, F.A.S. Mendonça, M. Santamaria-Jr, Electrical therapies act on the Ca2+/CaM signaling pathway to enhance bone regeneration with bioactive glass S53P4 and allogeneic grafts. J. Biomed. Mater. Res. B (2021). https://doi.org/10.1002/jbm.b.34858
M. Zayzafoon, Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. (2006). https://doi.org/10.1002/jcb.20675
M. Zayzafoon, K. Fulzele, J.M. McDonald, Calmodulin and calmodulin-dependent kinase IIalpha regulate osteoblast differentiation by controlling c-fos expression. J. Biol. Chem. (2005). https://doi.org/10.1074/jbc.M412680200
M.C. Farach-Carson, J.J. Bergh, Y. Xu, Integrating rapid responses to 1,25-dihydroxyvitamin D3 with transcriptional changes in osteoblasts: Ca2+ regulated pathways to the nucleus. Steroids (2004). https://doi.org/10.1016/j.steroids.2004.05.002
S. Wu, Q. Yu, A. Lai, J. Tian, Pulsed electromagnetic field induces Ca2+-dependent osteoblastogenesis in C3H10T1/2 mesenchymal cells through the Wnt-Ca2+/Wnt-β-catenin signaling pathway. Biochem. Biophys. Res. Commun. (2018). https://doi.org/10.1016/j.bbrc.2018.06.066
K. Maeda, Y. Kobayashi, M. Koide, S. Uehara, M. Okamoto, A. Ishihara, T. Kayama, M. Saito, K. Marumo, The regulation of bone metabolism and disorders by Wnt signaling. Int. J. Mol. Sci. (2019). https://doi.org/10.3390/ijms20225525
H. Miyamoto, Y. Sawaji, T. Iwaki, T. Masaoka, E. Fukada, M. Date, K. Yamamoto, Intermittent pulsed electromagnetic field stimulation activates the mTOR pathway and stimulates the proliferation of osteoblast-like cells. Bioelectromagnetics (2019). https://doi.org/10.1002/bem.22207
T.E. Patterson, Y. Sakai, M.D. Grabiner, M. Ibiwoye, R.J. Midura, M. Zborowski, A. Wolfman, Exposure of murine cells to pulsed electromagnetic fields rapidly activates the mTOR signaling pathway. Bioelectromagnetics (2006). https://doi.org/10.1002/bem.20244
M. Zhang, H. Jang, V. Gaponenko, R. Nussinov, Phosphorylated calmodulin promotes PI3K activation by binding to the SH2 domains. Biophys. J. (2017). https://doi.org/10.1016/j.bpj.2017.09.008
J. Chen, F. Long, mTORC1 signaling promotes osteoblast differentiation from preosteoblasts. PLoS ONE (2015). https://doi.org/10.1371/journal.pone.0130627
J. Chen, X. Tu, E. Esen, K.S. Joeng, C. Lin, J.M. Arbeit, M.A. Rüegg, M.N. Hall, L. Ma, F. Long, WNT7B promotes bone formation in part through mTORC1. PLoS Genet. (2014). https://doi.org/10.1371/journal.pgen.1004145
S. Sun, Y. Liu, S. Lipsky, M. Cho, Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. (2007). https://doi.org/10.1096/fj.06-7153com
E.C. Torre, M. Bicer, G.S. Cottrell, D. Widera, F. Tamagnini, Time-dependent reduction of calcium oscillations in adipose-derived stem cells differentiating towards adipogenic and osteogenic lineage. Biomolecules (2021). https://doi.org/10.3390/biom11101400
M.B. Bhavsar, G. Cato, A. Hauschild, L. Leppik, K.M. Costa Oliveira, M.J. Eischen-Loges, J.H. Barker, Membrane potential (Vmem) measurements during mesenchymal stem cell (MSC) proliferation and osteogenic differentiation. PeerJ (2019). https://doi.org/10.7717/peerj.6341
A.K. Harris, N.K. Pryer, D. Paydarfar, Effects of electric fields on fibroblast contractility and cytoskeleton. J. Exp. Zool. (1990). https://doi.org/10.1002/jez.1402530206
J. Ferrier, S.M. Ross, J. Kanehisa, J.E. Aubin, Osteoclasts and osteoblasts migrate in opposite directions in response to a constant electrical field. J. Cell. Physiol. (1986). https://doi.org/10.1002/jcp.1041290303
M.H. Lee, Y.J. Park, S.H. Hong, M.-A. Koo, M. Cho, J.-C. Park, Pulsed electrical stimulation enhances consistency of directional migration of adipose-derived stem cells. Cells (2021). https://doi.org/10.3390/cells10112846
N. Ozkucur, T.K. Monsees, S. Perike, H.Q. Do, R.H.W. Funk, Local calcium elevation and cell elongation initiate guided motility in electrically stimulated osteoblast-like cells. PLoS ONE (2009). https://doi.org/10.1371/journal.pone.0006131
M. Rohde, J. Ziebart, T. Kirschstein, T. Sellmann, K. Porath, F. Kühl, B. Delenda, C. Bahls, U. van Rienen, R. Bader, R. Köhling, Human osteoblast migration in DC electrical fields depends on store operated Ca2+-release and is correlated to upregulation of stretch-activated TRPM7 channels. Front. Bioeng. Biotechnol. (2019). https://doi.org/10.3389/fbioe.2019.00422
J. Zhang, M. Li, E.-T. Kang, K.G. Neoh, Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. (2016). https://doi.org/10.1016/j.actbio.2015.12.024
S. Mobini, Ü.-L. Talts, R. Xue, N.J. Cassidy, S.H. Cartmell, Electrical stimulation changes human mesenchymal stem cells orientation and cytoskeleton organization. J. Biomater. Tissue Eng. (2017). https://doi.org/10.1166/jbt.2017.1631
I. Titushkin, M. Cho, Regulation of cell cytoskeleton and membrane mechanics by electric field: role of linker proteins. Biophys. J. (2009). https://doi.org/10.1016/j.bpj.2008.09.035
B. Lin, S. Tsao, A. Chen, S.-K. Hu, L. Chao, P.G. Chao, Lipid rafts sense and direct electric field-induced migration. Proc. Natl. Acad. Sci. U.S.A. (2017). https://doi.org/10.1073/pnas.1702526114
J.U.A. Choi, A.W. Kijas, J. Lauko, A.E. Rowan, The mechanosensory role of osteocytes and implications for bone health and disease states. Front. Cell Dev. Biol. (2021). https://doi.org/10.3389/fcell.2021.770143
H. Ohshima, Bone loss and bone metabolism in astronauts during long-duration space flight. Clin. Calcium 16, 81–85 (2006)
J.L. Nourse, M.M. Pathak, How cells channel their stress: interplay between Piezo1 and the cytoskeleton. Semin. Cell Dev. Biol. (2017). https://doi.org/10.1016/j.semcdb.2017.06.018
N. Nabavi, A. Khandani, A. Camirand, R.E. Harrison, Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone (2011). https://doi.org/10.1016/j.bone.2011.07.036
J. Jin, R.T. Jaspers, G. Wu, J.A.M. Korfage, J. Klein-Nulend, A.D. Bakker, Shear stress modulates osteoblast cell and nucleus morphology and volume. Int. J. Mol. Sci. (2020). https://doi.org/10.3390/ijms21218361
F. Aglialoro, A. Abay, N. Yagci, M.A.E. Rab, L. Kaestner, R. van Wijk, M. von Lindern, E. van den Akker, Mechanical stress induces Ca2+-dependent signal transduction in erythroblasts and modulates erythropoiesis. Int. J. Mol. Sci. (2021). https://doi.org/10.3390/ijms22020955
X. Xu, S. Liu, H. Liu, K. Ru, Y. Jia, Z. Wu, S. Liang, Z. Khan, Z. Chen, A. Qian, L. Hu, Piezo channels: awesome mechanosensitive structures in cellular mechanotransduction and their role in bone. Int. J. Mol. Sci. (2021). https://doi.org/10.3390/ijms22126429
K. Saotome, S.E. Murthy, J.M. Kefauver, T. Whitwam, A. Patapoutian, A.B. Ward, Structure of the mechanically activated ion channel Piezo1. Nature (2018). https://doi.org/10.1038/nature25453
B. Coste, J. Mathur, M. Schmidt, T.J. Earley, S. Ranade, M.J. Petrus, A.E. Dubin, A. Patapoutian, Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science (New York, N.Y.) (2010). https://doi.org/10.1126/science.1193270
K. Poole, R. Herget, L. Lapatsina, H.-D. Ngo, G.R. Lewin, Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. (2014). https://doi.org/10.1038/ncomms4520
B.M. Gaub, D.J. Müller, Mechanical stimulation of Piezo1 receptors depends on extracellular matrix proteins and directionality of force. Nano Lett. (2017). https://doi.org/10.1021/acs.nanolett.7b00177
V. Maruthamuthu, B. Sabass, U.S. Schwarz, M.L. Gardel, Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc. Natl. Acad. Sci. U.S.A. (2011). https://doi.org/10.1073/pnas.1011123108
S.K. Sastry, K. Burridge, Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. (2000). https://doi.org/10.1006/excr.2000.5043
R.O. Hynes, Integrins: versatility, modulation, and signaling in cell adhesion. Cell (1992). https://doi.org/10.1016/0092-8674(92)90115-s
I.D. Campbell, M.J. Humphries, Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. (2011). https://doi.org/10.1101/cshperspect.a004994
N. Wang, D.E. Ingber, Probing transmembrane mechanical coupling and cytomechanics using magnetic twisting cytometry. Biochem. Cell Biol. (1995). https://doi.org/10.1139/o95-041
K.L. Mui, C.S. Chen, R.K. Assoian, The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J. Cell Sci. (2016). https://doi.org/10.1242/jcs.183699
P. Müller, A. Langenbach, A. Kaminski, J. Rychly, Modulating the actin cytoskeleton affects mechanically induced signal transduction and differentiation in mesenchymal stem cells. PLoS ONE (2013). https://doi.org/10.1371/journal.pone.0071283
C. Schmidt, H. Pommerenke, F. Dürr, B. Nebe, J. Rychly, Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J. Biol. Chem. (1998). https://doi.org/10.1074/jbc.273.9.5081
H. Pommerenke, C. Schmidt, F. Dürr, B. Nebe, F. Lüthen, P. Muller, J. Rychly, The mode of mechanical integrin stressing controls intracellular signaling in osteoblasts. J. Bone Miner. Res. (2002). https://doi.org/10.1359/jbmr.2002.17.4.603
B. Nebe, J. Rychly, A. Knopp, W. Bohn, Mechanical induction of beta 1-integrin-mediated calcium signaling in a hepatocyte cell line. Exp. Cell Res. (1995). https://doi.org/10.1006/excr.1995.1181
D. Zhao, R. Hua, M.A. Riquelme, H. Cheng, T. Guda, H. Xu, S. Gu, J.X. Jiang, Osteocytes regulate bone anabolic response to mechanical loading in male mice via activation of integrin α5. Bone Res. (2022). https://doi.org/10.1038/s41413-022-00222-z
H. Pommerenke, E. Schreiber, F. Dürr, B. Nebe, C. Hahnel, W. Möller, J. Rychly, Stimulation of integrin receptors using a magnetic drag force device induces an intracellular free calcium response. Eur. J. Cell Biol. 70, 157–164 (1996)
K.S. Zeller, A. Riaz, H. Sarve, J. Li, A. Tengholm, S. Johansson, The role of mechanical force and ROS in integrin-dependent signals. PLoS ONE (2013). https://doi.org/10.1371/journal.pone.0064897
M.L. Taddei, M. Parri, T. Mello, A. Catalano, A.D. Levine, G. Raugei, G. Ramponi, P. Chiarugi, Integrin-mediated cell adhesion and spreading engage different sources of reactive oxygen species. Antioxid. Redox Signal. (2007). https://doi.org/10.1089/ars.2006.1392
N. Wang, Mechanical interactions among cytoskeletal filaments. Hypertension (Dallas, Tex.: 1979) (1998). https://doi.org/10.1161/01.HYP.32.1.162
B. Nebe, C. Holzhausen, J. Rychly, W. Urbaszek, Impaired mechanisms of leukocyte adhesion in vitro by the calcium channel antagonist mibefradil. Cardiovasc. Drugs Ther. (2002). https://doi.org/10.1023/A:1020688019792
K. Peter, T.E. O’Toole, Modulation of cell adhesion by changes in alpha L beta 2 (LFA-1, CD11a/CD18) cytoplasmic domain/cytoskeleton interaction. J. Exp. Med. (1995). https://doi.org/10.1084/jem.181.1.315
C.G. Galbraith, K.M. Yamada, M.P. Sheetz, The relationship between force and focal complex development. J. Cell Biol. (2002). https://doi.org/10.1083/jcb.200204153
S. Yonemura, Y. Wada, T. Watanabe, A. Nagafuchi, M. Shibata, Alpha-catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. (2010). https://doi.org/10.1038/ncb2055
J.R. Lange, B. Fabry, Cell and tissue mechanics in cell migration. Exp. Cell Res. (2013). https://doi.org/10.1016/j.yexcr.2013.04.023
B. Fabry, A.H. Klemm, S. Kienle, T.E. Schäffer, W.H. Goldmann, Focal adhesion kinase stabilizes the cytoskeleton. Biophys. J. (2011). https://doi.org/10.1016/j.bpj.2011.09.043
S. Vermeulen, Z. Tahmasebi Birgani, P. Habibovic, Biomaterial-induced pathway modulation for bone regeneration. Biomaterials (2022). https://doi.org/10.1016/j.biomaterials.2022.121431
H. Rebl, B. Finke, K. Schroeder, J.B. Nebe, Time-dependent metabolic activity and adhesion of human osteoblast-like cells on sensor chips with a plasma polymer nanolayer. Int. J. Artif. Organs 33, 738–748 (2010)
G. Fridman, G. Friedman, A. Gutsol, A.B. Shekhter, V.N. Vasilets, A. Fridman, Applied plasma medicine. Plasma Process. Polym. (2008). https://doi.org/10.1002/ppap.200700154
G. Isbary, T. Shimizu, J.L. Zimmermann, H.M. Thomas, G.E. Morfill, W. Stolz, Cold atmospheric plasma for local infection control and subsequent pain reduction in a patient with chronic post-operative ear infection. New Microbes New Infect. (2013). https://doi.org/10.1002/2052-2975.19
H. Conrads, M. Schmidt, Plasma generation and plasma sources. Plasma Sources Sci. Technol. (2000). https://doi.org/10.1088/0963-0252/9/4/301
X. Lu, G.V. Naidis, M. Laroussi, S. Reuter, D.B. Graves, K. Ostrikov, Reactive species in non-equilibrium atmospheric-pressure plasmas: generation, transport, and biological effects. Phys. Rep. (2016). https://doi.org/10.1016/j.physrep.2016.03.003
K.-D. Weltmann, E. Kindel, R. Brandenburg, C. Meyer, R. Bussiahn, C. Wilke, T. von Woedtke, Atmospheric pressure plasma jet for medical therapy: plasma parameters and risk estimation. Contrib. Plasma Phys. (2009). https://doi.org/10.1002/ctpp.200910067
J. Gay-Mimbrera, M.C. García, B. Isla-Tejera, A. Rodero-Serrano, A.V. García-Nieto, J. Ruano, Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv. Ther. (2016). https://doi.org/10.1007/s12325-016-0338-1
S. Reuter, T. von Woedtke, K.-D. Weltmann, The kINPen—a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D (2018). https://doi.org/10.1088/1361-6463/aab3ad
X. Dai, Z. Zhang, J. Zhang, K. Ostrikov, Dosing: the key to precision plasma oncology. Plasma Process. Polym. (2020). https://doi.org/10.1002/ppap.201900178
H. Rebl, C. Bergemann, S. Rakers, B. Nebe, A. Rebl, Plasma treatment of fish cells: the importance of defining cell culture conditions in comparative studies. Appl. Sci. (2021). https://doi.org/10.3390/app11062534
E. Biscop, A. Lin, W. van Boxem, J. van Loenhout, J. de Backer, C. Deben, S. Dewilde, E. Smits, A.A. Bogaerts, Influence of cell type and culture medium on determining cancer selectivity of cold atmospheric plasma treatment. Cancers (2019). https://doi.org/10.3390/cancers11091287
E. Simoncelli, A. Stancampiano, M. Boselli, M. Gherardi, V. Colombo, Experimental investigation on the influence of target physical properties on an impinging plasma jet. Plasma (2019). https://doi.org/10.3390/plasma2030029
S. Kalghatgi, C.M. Kelly, E. Cerchar, B. Torabi, O. Alekseev, A. Fridman, G. Friedman, J. Azizkhan-Clifford, Effects of non-thermal plasma on mammalian cells. PLoS ONE (2011). https://doi.org/10.1371/journal.pone.0016270
T. Bernhardt, M.L. Semmler, M. Schäfer, S. Bekeschus, S. Emmert, L. Boeckmann, Plasma medicine: applications of cold atmospheric pressure plasma in dermatology. Oxid. Med. Cell. Longev. (2019). https://doi.org/10.1155/2019/3873928
T. Nosenko, T. Shimizu, G.E. Morfill, Designing plasmas for chronic wound disinfection. New J. Phys. (2009). https://doi.org/10.1088/1367-2630/11/11/115013
S.R. Lipner, G. Friedman, R.K. Scher, Pilot study to evaluate a plasma device for the treatment of onychomycosis. Clin. Exp. Dermatol. (2017). https://doi.org/10.1111/ced.12973
C.-H. Kim, S. Kwon, J.H. Bahn, K. Lee, S.I. Jun, P.D. Rack, S.J. Baek, Effects of atmospheric nonthermal plasma on invasion of colorectal cancer cells. Appl. Phys. Lett. (2010). https://doi.org/10.1063/1.3449575
C. Schneider, L. Gebhardt, S. Arndt, S. Karrer, J.L. Zimmermann, M.J.M. Fischer, A.-K. Bosserhoff, Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence. Sci. Rep. (2018). https://doi.org/10.1038/s41598-018-28443-5
S.U. Kang, J.-H. Cho, J.W. Chang, Y.S. Shin, K.I. Kim, J.K. Park, S.S. Yang, J.-S. Lee, E. Moon, K. Lee, C.-H. Kim, Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. (2014). https://doi.org/10.1038/cddis.2014.33
S.N. Zucker, J. Zirnheld, A. Bagati, T.M. DiSanto, B. Des Soye, J.A. Wawrzyniak, K. Etemadi, M. Nikiforov, R. Berezney, Preferential induction of apoptotic cell death in melanoma cells as compared with normal keratinocytes using a non-thermal plasma torch. Cancer Biol. Ther. (2012). https://doi.org/10.4161/cbt.21787
H. Rebl, M. Sawade, M. Hein, C. Bergemann, M. Wende, M. Lalk, P. Langer, S. Emmert, B. Nebe, Synergistic effect of plasma-activated medium and novel indirubin derivatives on human skin cancer cells by activation of the AhR pathway. Sci. Rep. (2022). https://doi.org/10.1038/s41598-022-06523-x
F. Faramarzi, P. Zafari, M. Alimohammadi, M. Moonesi, A. Rafiei, S. Bekeschus, Cold physical plasma in cancer therapy: mechanisms, signaling, and immunity. Oxid. Med. Cell. Longev. (2021). https://doi.org/10.1155/2021/9916796
M. Keidar, A. Shashurin, O. Volotskova, M. Ann Stepp, P. Srinivasan, A. Sandler, B. Trink, Cold atmospheric plasma in cancer therapy. Phys. Plasmas (2013). https://doi.org/10.1063/1.4801516
A.M. Hirst, F.M. Frame, M. Arya, N.J. Maitland, D. O’Connell, Low temperature plasmas as emerging cancer therapeutics: the state of play and thoughts for the future. Tumour Biol. (2016). https://doi.org/10.1007/s13277-016-4911-7
P. García-Nogales, A. Almeida, J.P. Bolaños, Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J. Biol. Chem. (2003). https://doi.org/10.1074/jbc.M206835200
J.-Y. Paik, K.-H. Jung, J.-H. Lee, J.-W. Park, K.-H. Lee, Reactive oxygen species-driven HIF1α triggers accelerated glycolysis in endothelial cells exposed to low oxygen tension. Nucl. Med. Biol. (2017). https://doi.org/10.1016/j.nucmedbio.2016.10.006
S.J. Forrester, D.S. Kikuchi, M.S. Hernandes, Q. Xu, K.K. Griendling, Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. (2018). https://doi.org/10.1161/CIRCRESAHA.117.311401
A. Roveri, M. Coassin, M. Maiorino, A. Zamburlini, F.T. van Amsterdam, E. Ratti, F. Ursini, Effect of hydrogen peroxide on calcium homeostasis in smooth muscle cells. Arch. Biochem. Biophys. (1992). https://doi.org/10.1016/0003-9861(92)90671-i
A. Görlach, K. Bertram, S. Hudecova, O. Krizanova, Calcium and ROS: a mutual interplay. Redox Biol. (2015). https://doi.org/10.1016/j.redox.2015.08.010
L. Wu, W. Lian, L. Zhao, Calcium signaling in cancer progression and therapy. FEBS J. (2021). https://doi.org/10.1111/febs.16133
R. Ishii, T. Kamiya, H. Hara, T. Adachi, Hyperthermia synergistically enhances cancer cell death by plasma-activated acetated Ringer’s solution. Arch. Biochem. Biophys. (2020). https://doi.org/10.1016/j.abb.2020.108565
M. Keidar, R. Walk, A. Shashurin, P. Srinivasan, A. Sandler, S. Dasgupta, R. Ravi, R. Guerrero-Preston, B. Trink, Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer (2011). https://doi.org/10.1038/bjc.2011.386
W. Hu, J.J. Kavanagh, Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. (2003). https://doi.org/10.1016/s1470-2045(03)01277-4
J. Vásquez-Vivar, P. Martasek, N. Hogg, B.S. Masters, K.A. Pritchard, B. Kalyanaraman, Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry (1997). https://doi.org/10.1021/bi971475e
D. Yan, A. Talbot, N. Nourmohammadi, J.H. Sherman, X. Cheng, M. Keidar, Toward understanding the selective anticancer capacity of cold atmospheric plasma—a model based on aquaporins (review). Biointerphases (2015). https://doi.org/10.1116/1.4938020
S. Bekeschus, G. Liebelt, J. Menz, J. Berner, S.K. Sagwal, K. Wende, K.-D. Weltmann, L. Boeckmann, T. von Woedtke, H.-R. Metelmann, S. Emmert, A. Schmidt, Tumor cell metabolism correlates with resistance to gas plasma treatment: the evaluation of three dogmas. Free Radic. Biol. Med. (2021). https://doi.org/10.1016/j.freeradbiomed.2021.02.035
D. Trachootham, J. Alexandre, P. Huang, Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. (2009). https://doi.org/10.1038/nrd2803
G. Bauer, Tumor cell-protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res. 32, 2599–2624 (2012)
J. van der Paal, S.-H. Hong, M. Yusupov, N. Gaur, J.-S. Oh, R.D. Short, E.J. Szili, A. Bogaerts, How membrane lipids influence plasma delivery of reactive oxygen species into cells and subsequent DNA damage: an experimental and computational study. Phys. Chem. Chem. Phys. (2019). https://doi.org/10.1039/C9CP03520F
M. Hoentsch, T. von Woedtke, K.-D. Weltmann, J. Barbara Nebe, Time-dependent effects of low-temperature atmospheric-pressure argon plasma on epithelial cell attachment, viability and tight junction formation in vitro. J. Phys. D (2012). https://doi.org/10.1088/0022-3727/45/2/025206
C. Bergemann, T. Gerling, C. Hoppe, M. Karmazyna, M. Höntsch, M. Eggert, B. Nebe, Physicochemical analysis of argon plasma-treated cell culture medium, in Plasma Science and Technology—Progress in Physical States and Chemical Reactions. ed. by T. Mieno (IntechOpen, Berlin, 2016)
I. Adamovich, S. Agarwal, E. Ahedo, L.L. Alves, S. Baalrud, N. Babaeva, A. Bogaerts, A. Bourdon, P.J. Bruggeman, C. Canal, E.H. Choi, S. Coulombe, Z. Donkó, D.B. Graves, S. Hamaguchi, D. Hegemann, M. Hori, H.-H. Kim, G.M.W. Kroesen, M.J. Kushner, A. Laricchiuta, X. Li, T.E. Magin, S. Mededovic Thagard, V. Miller, A.B. Murphy, G.S. Oehrlein, N. Puac, R.M. Sankaran, S. Samukawa, M. Shiratani, M. Šimek, N. Tarasenko, K. Terashima, E. Thomas Jr., J. Trieschmann, S. Tsikata, M.M. Turner, I.J. van der Walt, M.C.M. van de Sanden, T. von Woedtke, The 2022 Plasma Roadmap: low temperature plasma science and technology. J. Phys. D (2022). https://doi.org/10.1088/1361-6463/ac5e1c
M. Rasouli, N. Fallah, S. Bekeschus, Combining nanotechnology and gas plasma as an emerging platform for cancer therapy: mechanism and therapeutic implication. Oxid. Med. Cell. Longev. (2021). https://doi.org/10.1155/2021/2990326
N.B. Lipko, Photobiomodulation: evolution and adaptation. Photobiomodul. Photomed. Laser Surg. (2022). https://doi.org/10.1089/photob.2021.0145
L.F. de Freitas, M.R. Hamblin, Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. (2016). https://doi.org/10.1109/JSTQE.2016.2561201
H. Serrage, V. Heiskanen, W.M. Palin, P.R. Cooper, M.R. Milward, M. Hadis, M.R. Hamblin, Under the spotlight: mechanisms of photobiomodulation concentrating on blue and green light. Photochem. Photobiol. Sci. (2019). https://doi.org/10.1039/c9pp00089e
T.I. Karu, Cellular and molecular mechanisms of photobiomodulation (low-power laser therapy). IEEE J. Sel. Top. Quantum Electron. (2014). https://doi.org/10.1109/JSTQE.2013.2273411
R.O. Poyton, K.A. Ball, Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov. Med. 11, 154–159 (2011)
B. Quirk, H.T. Whelan, Effect of red-to-near infrared light and a nitric oxide donor on the oxygen consumption of isolated cytochrome C oxidase. Photobiomodul. Photomed. Laser Surg. (2021). https://doi.org/10.1089/photob.2020.4978
B.J. Quirk, H.T. Whelan, What lies at the heart of photobiomodulation: light, cytochrome C oxidase, and nitric oxide-review of the evidence. Photobiomodul. Photomed. Laser Surg. (2020). https://doi.org/10.1089/photob.2020.4905
A.P. Sommer, Mitochondrial cytochrome C oxidase is not the primary acceptor for near infrared light-it is mitochondrial bound water: the principles of low-level light therapy. Ann. Transl. Med. (2019). https://doi.org/10.21037/atm.2019.01.43
K. Khorsandi, R. Hosseinzadeh, H. Abrahamse, R. Fekrazad, Biological responses of stem cells to photobiomodulation therapy. Curr. Stem Cell Res. Ther. (2020). https://doi.org/10.2174/1574888X15666200204123722
I.L. Zungu, D. Hawkins Evans, H. Abrahamse, Mitochondrial responses of normal and injured human skin fibroblasts following low level laser irradiation—an in vitro study. Photochem. Photobiol. (2009). https://doi.org/10.1111/j.1751-1097.2008.00523.x
N. Hakimiha, M.M. Dehghan, H. Manaheji, J. Zaringhalam, S. Farzad-Mohajeri, R. Fekrazad, N. Moslemi, Recovery of inferior alveolar nerve by photobiomodulation therapy using two laser wavelengths: a behavioral and immunological study in rat. J. Photochem. Photobiol. B (2020). https://doi.org/10.1016/j.jphotobiol.2020.111785
S. Barreto Ortiz, D. Hori, Y. Nomura, X. Yun, H. Jiang, H. Yong, J. Chen, S. Paek, D. Pandey, G. Sikka, A. Bhatta, A. Gillard, J. Steppan, J.H. Kim, H. Adachi, V.M. Barodka, L. Romer, S.S. An, L.A. Shimoda, L. Santhanam, D.E. Berkowitz, Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. (2018). https://doi.org/10.1152/ajplung.00091.2017
I. Castellano-Pellicena, N.E. Uzunbajakava, C. Mignon, B. Raafs, V.A. Botchkarev, M.J. Thornton, Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med. (2019). https://doi.org/10.1002/lsm.23015
K. Haltaufderhyde, R.N. Ozdeslik, N.L. Wicks, J.A. Najera, E. Oancea, Opsin expression in human epidermal skin. Photochem. Photobiol. (2015). https://doi.org/10.1111/php.12354
K. Ondrusova, M. Fatehi, A. Barr, Z. Czarnecka, W. Long, K. Suzuki, S. Campbell, K. Philippaert, M. Hubert, E. Tredget, P. Kwan, N. Touret, M. Wabitsch, K.Y. Lee, P.E. Light, Subcutaneous white adipocytes express a light sensitive signaling pathway mediated via a melanopsin/TRPC channel axis. Sci. Rep. (2017). https://doi.org/10.1038/s41598-017-16689-4
E. Austin, A.N. Geisler, J. Nguyen, I. Kohli, I. Hamzavi, H.W. Lim, J. Jagdeo, Visible light. Part I: properties and cutaneous effects of visible light. J. Am. Acad. Dermatol. (2021). https://doi.org/10.1016/j.jaad.2021.02.048
A. Stachurska, T. Sarna, Regulation of melanopsin signaling: key interactions of the nonvisual photopigment. Photochem. Photobiol. (2019). https://doi.org/10.1111/php.12995
Q. Gu, L. Wang, F. Huang, W. Schwarz, Stimulation of TRPV1 by green laser light. Evid. Based Complement. Altern. Med. (2012). https://doi.org/10.1155/2012/857123
R. Sakaguchi, Y. Mori, Transient receptor potential (TRP) channels: biosensors for redox environmental stimuli and cellular status. Free Radic. Biol. Med. (2020). https://doi.org/10.1016/j.freeradbiomed.2019.10.415
A. Amaroli, S. Ravera, A. Zekiy, S. Benedicenti, C. Pasquale, A narrative review on oral and periodontal bacteria microbiota photobiomodulation, through visible and near-infrared light: from the origins to modern therapies. Int. J. Mol. Sci. (2022). https://doi.org/10.3390/ijms23031372
M.-Y. Yang, C.-J. Chang, L.-Y. Chen, Blue light induced reactive oxygen species from flavin mononucleotide and flavin adenine dinucleotide on lethality of HeLa cells. J. Photochem. Photobiol. B (2017). https://doi.org/10.1016/j.jphotobiol.2017.06.014
P.R. Arany, A. Cho, T.D. Hunt, G. Sidhu, K. Shin, E. Hahm, G.X. Huang, J. Weaver, A.C.-H. Chen, B.L. Padwa, M.R. Hamblin, M.H. Barcellos-Hoff, A.B. Kulkarni, D.J. Mooney, Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. (2014). https://doi.org/10.1126/scitranslmed.3008234
A. Amat, J. Rigau, R.W. Waynant, I.K. Ilev, J. Tomas, J.J. Anders, Modification of the intrinsic fluorescence and the biochemical behavior of ATP after irradiation with visible and near-infrared laser light. J. Photochem. Photobiol. B (2005). https://doi.org/10.1016/j.jphotobiol.2005.05.012
C. Ash, M. Dubec, K. Donne, T. Bashford, Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. (2017). https://doi.org/10.1007/s10103-017-2317-4
D. Barolet, Light-emitting diodes (LEDs) in dermatology. Semin. Cutan. Med. Surg. (2008). https://doi.org/10.1016/j.sder.2008.08.003
H. Pinto, P. Goñi Oliver, E. Sánchez-Vizcaíno Mengual, The effect of photobiomodulation on human mesenchymal cells: a literature review. Aesthet. Plast. Surg. (2021). https://doi.org/10.1007/s00266-021-02173-y
P. Samadi, S. Saki, H. Manoochehri, M. Sheykhhasan, Therapeutic applications of mesenchymal stem cells: a comprehensive review. Curr. Stem Cell Res. Ther. (2021). https://doi.org/10.2174/1574888X15666200914142709
A. Tani, F. Chellini, M. Giannelli, D. Nosi, S. Zecchi-Orlandini, C. Sassoli, Red (635 nm), near-infrared (808 nm) and violet-blue (405 nm) photobiomodulation potentiality on human osteoblasts and mesenchymal stromal cells: a morphological and molecular in vitro study. Int. J. Mol. Sci. (2018). https://doi.org/10.3390/ijms19071946
J.M. Miranda, J.A.A. de Arruda, L.M.M. Moreno, W.D.C. Gaião, S.V.B. do Nascimento, E.V. de Souza Silva, M.B. da Silva, C.G. Rodrigues, D.S. de Albuquerque, R. Braz, A.L.B. Pinheiro, M.E.M. de Martinez Gerbi, Photobiomodulation therapy in the proliferation and differentiation of human umbilical cord mesenchymal stem cells: an in vitro study. J. Lasers Med. Sci. (2020). https://doi.org/10.34172/jlms.2020.73
H. Chen, H. Wu, H. Yin, J. Wang, H. Dong, Q. Chen, Y. Li, Effect of photobiomodulation on neural differentiation of human umbilical cord mesenchymal stem cells. Lasers Med. Sci. (2019). https://doi.org/10.1007/s10103-018-2638-y
W.-K. Ong, H.-F. Chen, C.-T. Tsai, Y.-J. Fu, Y.-S. Wong, D.-J. Yen, T.-H. Chang, H.-D. Huang, O.K.-S. Lee, S. Chien, J.H.-C. Ho, The activation of directional stem cell motility by green light-emitting diode irradiation. Biomaterials (2013). https://doi.org/10.1016/j.biomaterials.2012.11.065
M.R. Love, S. Palee, S.C. Chattipakorn, N. Chattipakorn, Effects of electrical stimulation on cell proliferation and apoptosis. J. Cell. Physiol. (2018). https://doi.org/10.1002/jcp.25975
K.M. Holmström, T. Finkel, Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. (2014). https://doi.org/10.1038/nrm3801
A.V. Gordeeva, R.A. Zvyagilskaya, Y.A. Labas, Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry (2003). https://doi.org/10.1023/a:1026398310003
Acknowledgements
This work was kindly financially supported by (i) the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—SFB 1270/1,2—299150580 (ELAINE); as well as CRC TR37/2; (ii) the Federal Ministry of Education and Research (BMBF, No. 13N9775 and 13N11183, Campus PlasmaMed); (iii) the European Social Fund (ESF), Reference No. ESF/14-BM-A55-0002/18 (ONKOTHER-H) and the Ministry of Education, Science and Culture of Mecklenburg-West Pomerania, Germany; and (iv) the European Regional Development Fund, No. TBI-V-1-336-VBW-116 (ActiHeal). We thank all our project partners for fruitful discussions and inspirational cooperations.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bielfeldt, M., Rebl, H., Peters, K. et al. Sensing of Physical Factors by Cells: Electric Field, Mechanical Forces, Physical Plasma and Light—Importance for Tissue Regeneration. Biomedical Materials & Devices 1, 146–161 (2023). https://doi.org/10.1007/s44174-022-00028-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-022-00028-x