Abstract
With the growing concern about human health issues, especially during the outbreak of the COVID-19 pandemic, the demand for personalized healthcare regarding disease prevention and recovery is increasing. However, tremendous challenges lie in both limited public medical resources and costly medical diagnosis approaches. Recently, skin-attachable sensors have emerged as promising health monitoring platforms to overcome such difficulties. Owing to the advantages of good comfort and high signal-to-noise ratio, skin-attachable sensors enable household, real-time, and long-term detection of weak physiological signals to efficiently and accurately monitor human motion, heart rate, blood oxygen saturation, respiratory rate, lung and heart sound, glucose, and biomarkers in biomedical applications. To further improve the integration level of biomedical skin-attachable sensors, efforts have been made in combining multiple sensing techniques with elaborate structural designs. This review summarizes the recent advances in different functional skin-attachable sensors, which monitor physical and chemical indicators of the human body. The advantages, shortcomings, and integration strategies of different mechanisms are presented. Specially, we highlight sensors monitoring pulmonary function such as respiratory rate and blood oxygen saturation for their potential usage in the COVID-19 pandemic. Finally, the future development of skin-attachable sensors is envisioned.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rise of mobile health and the increase in people's health awareness, intelligent and continuous health monitoring is of great significance in improving the standard of living, disease prevention, and monitoring during the rehabilitation process. Meanwhile, the outbreak of the COVID-19 pandemic requires real-time physiological monitoring such as respiratory rate for early diagnosis due to its fast spreading [1,2,3]. Traditional medical instruments in the hospital pose challenges under such requirements, since they are complicated to operate and can hardly realize continuous monitoring. Health monitoring functions are thus integrated into commercial wearable devices, such as smart watches and headbands [4], enabling real-time detection and online analysis. However, currently available wearable devices are rigid devices that cannot conformally fit the skin, causing discomfort during long-term usage and inevitable signal noise from the surroundings. The idea of skin-attachable sensors is therefore proposed and has become one of the hottest research fields in recent years. Skin-attachable sensors feature unprecedented wearing comfort and data reliability by utilizing biocompatible and flexible materials with ultrathin and stretchable structural designs. These advantages make them important in household, real-time, and long-term health monitoring and disease diagnosis [5,6,7,8,9].
Skin-attachable sensors mainly include physical and chemical sensors in biomedical applications (Fig. 1). Physical sensors are based on electrical and optical mechanisms to measure physiological signals, including motion, heart rate, oxygen saturation, respiratory rate, and lung and heart sound. Motion sensors are usually used to monitor human behavior [10,11,12]. In particular, the monitoring of body posture can effectively reduce the risk of falls and assess the behavioral disorders of the elderly [13, 14]. Skin-attachable heart rate, respiration, and oxygen saturation sensors provide real-time monitoring of cardiovascular and respiratory disease conditions [15]. The early diagnosis of cardiovascular and cardiopulmonary issues through skin-attachable sensors is significant considering their high mortality rate and high correlation with the symptoms of the COVID-19. Traditional chemical sensors require invasive acquisition of blood to evaluate the concentration of glucose and other biomarkers in human body. Skin-attachable chemical sensors are mostly based on electrochemical and optical mechanisms, which enable non-invasive real-time monitoring through body fluids. For diabetic patients, skin-attachable chemical sensors can provide real-time feedback on the patient's blood glucose level, facilitating their self-management [16, 17].
Monitoring indicators of skin-attachable sensors for biomedical applications. Telemedicine. Reprinted with permission from Ref. [8]. Copyright 2020 American Chemical Society. Health monitoring. Reprinted with permission from Ref. [7]. Copyright 2022 Elsevier. Disease diagnosis. Reprinted with permission from Ref. [9]. Copyright 2020 Elsevier
Nevertheless, to outperform traditional biomedical devices in practical applications, challenges arise when balancing wearability and functionality. To this end, researchers have focused on both material and structural optimizations in recent skin-attachable sensors. Nanomaterials and conductive polymers could be easily fabricated into thin films or wires and maintain stable electrical and mechanical characteristics under strain, making them ideal functional sensory components [18,19,20]. Structurally, the integration of multiple functions to collect comprehensive biomedical information while minimizing device complexity is favored. In addition, wireless powering and self-powering techniques are also leveraged to reduce the power consumption, achieving improved durability and portability [21, 22]. In this review, we will introduce the advances of skin-attachable sensors from the perspective of sensor functions including novel ones for use in the COVID-19 pandemic, as well as considerations in choosing different sensing sensory materials, structures, and sensing mechanisms. First, we introduce sensors that monitor the physical signals of the human body, such as human motion, heart rate, and respiratory rate. Next, chemical sensors are summarized, including glucose and other biomarkers that reflect human health status. After that, methods of implementation and research progress of multifunctional sensors are presented. Finally, the potential research directions for skin-attachable sensors are prospected.
Physical Sensor
Physical sensors have been applicated in various biomedical fields, including the monitoring of human motion, heart rate, oxygen saturation (SpO2), respiratory rate, and lung and heart sound. By converting the changes of physical quantities such as mechanical deformation and temperature into electrical signals, key health status indicators can be acquired and long-term monitored.
Motion
Human motion monitoring plays an important role in sports and disease risk appraisal. It can obtain data to help assess the physical activity and sports posture of athletes. For elderly individuals, motion monitoring is valuable in estimating their falling risk and providing timely alerts on their physiological state. Human motions usually cause changes in stress and strain on body. Skin-attachable strain sensors are, therefore, suitable for long-term motion monitoring. The measurement mechanisms of the sensors are mainly piezoresistive, piezoelectric, and triboelectric mechanisms. Sensitivity, response range, and resistance to environment are important indicators for skin-attachable motion sensors.
For piezoresistive sensors, sensitivity and stretchability are often conflicting. Hybrid structural design can solve performance problems of piezoresistive sensors [23]. Chen et al. [24] used freeze and drying thermal imidization techniques to fabricate a polyimide (PI)/carbon nanotube (CNT) composite aerogel sensor with ultrahigh sensitivity of 11.28 kPa−1, wide sensing range of 80% strain, and long-term stability for 1000 cycles (Fig. 2a). Combining the advantages of PI and CNT, the proposed device can be applied to monitor finger movements and walking and other motions. He et al. [25] developed a fiber sensor based on multi-walled carbon nanotube (MWCNT) and thermoplastic polyurethanes (TPU). This fiber sensor achieved high stretchability and sensitivity by combining the merits of TPU and MWCNT sensing unit.
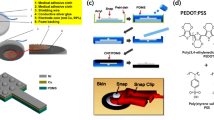
a Optical images of PI/CNT composite aerogels and ΔR/R0 as a function of compressive stress. The fabricated skin-attachable sensor responded with high sensitivity to pressure. Reprint with permission from Ref. [24]. Copyright 2019 American Chemical Society. b Structure schematic diagram of STMES. Reprint with permission from Ref. [27]. Copyright 2022 Elsevier. c Schematic diagram of Al/PEDOT:PSS Schottky contact. Reprint with permission from Ref. [32]. Copyright 2021 John Wiley & Sons
Triboelectric sensors can be used as self-powered sensors but are susceptible to environmental conditions. The structural design and material selection of the triboelectric layer are critical. Dong et al. [26] combined conventional textiles with triboelectric nanogenerators (TENG). Through a three-dimensional five-directional braided structure, a 3D braided TENG was designed to improve the output and sensing capability of the triboelectric sensor. Zhang et al. [27] used silver nanowires and PDMS to make a contact-separated TENG with a mechanoluminescent layer, proposing a self-powered triboelectric-mechanoluminescent electronic skin (STMES) that can detect human mechanical motion using electrical and optical signals without additional power supply (Fig. 2b). Wang et al. [28] used polyacrylamide/polyacrylic acid/graphene/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) hydrogel incorporated with CNTs and polydimethylsiloxane (PDMS) to fabricate TENG, which can be used to monitor human activities and harvest energy from human motion. Piezoelectric sensors mostly require inorganic materials to synthesize piezoelectric composites to improve their performance, which is still a major challenge. The shortcomings of the single mechanism are expected to be overcome through a combination of mechanisms, such as piezoelectric and triboelectric [29].
Compared to piezoelectric and triboelectric self-powered sensors, recently developed sensors based on Schottky contact can directly convert mechanical stimuli into DC electrical outputs without additional rectifiers, which is more conducive to the miniaturization of sensors [30]. Yang et al. [31] fabricated a self-powered sensor based on polypyrrole (PPy)/Al Schottky contact. The sensor adopted a sandwich structure with three layers, respectively, PPy-coated fabric, Ni-coated fabric, and Al plastic film. This work indicates the potential application of Schottky contact for human motion sensing. Liu et al. [32] proposed a flexible Schottky generator based on Al/PEDOT:PSS Schottky contact, which is expected to be applied in skin-attachable sensors (Fig. 2c).
Heart Rate
Long-term monitoring of cardiovascular signals, such as heart rate (HR), is crucial for the early prevention and screening of cardiovascular disease which has a high mortality rate [15, 33]. Traditional monitoring devices such as electrocardiogram monitor require professionals to operate. Moreover, frequent and long-term contact between electrodes and skin can cause cutaneous anaphylaxis [34]. Skin-attachable sensors that can be worn comfortably for long periods of time are gaining widespread interest. Currently, skin-attachable heart rate sensing is mainly performed via bioelectric (e.g., electrocardiography) and photoelectric approaches (e.g., photoplethysmography). In addition, strain and pressure sensors can also enable HR monitoring in joint sites [35].
The most popular bioelectrical method is to obtain electrocardiogram (ECG) by electrocardiography. ECG is periodic signals where the heart rate can be derived from the interval of R-R peaks. When collecting ECG signals, the biocompatibility and skin contact impedance of electrodes are critical factors that influence the performance of the sensors. Conventionally, there are dry and wet electrodes, but they affect wearing comfort to varying degrees. Wet electrodes need conductive gel to fit the skin, which can cause allergies. Dry electrodes fit poorly to the skin and obtain ECG signals with low signal-to-noise ratio (SNR). Electrodes with biocompatible materials as substrate and conformal serpentine or kirigami conductive network designs can overcome the dilemma between wearing comfort and contact impedance of conventional electrodes. Wang et al. [36] used cellulose and polyvinyl alcohol as substrates to design flexible polyvinyl alcohol/cellulose/PEDOT:PSS(PCPP) composite electrode (Fig. 3a). By adjusting the 3,4-ethoxylene dioxy thiophene (EDOT) content, the PCPP film can obtain clear ECG signals with excellent flexibility. Won et al. [37] utilized kirigami approach to realize transparent stretchable electrodes, which can achieve strains of over 400%.
The photoelectric method is usually photoplethysmography (PPG). Since heart activity causes changes in arterial blood volume, the intensity of the transmitted or reflected light changed accordingly, which can be detected to monitor the heart rate. Although PPG heart rate sensors consisting of a photodetector (PD) and several pairs of light-emitting diodes (LEDs) are now commercially available [38], the performance of PD imposes restrictions on the improvement of wearable PPG heart rate sensors in broader application scenarios. To achieve better photoresponse of flexible PDs, Simões et al. [39] proposed a flexible organic photodetector (OPD) by incorporating a bulk heterojunction and integrating PEDOT:PSS and perylene diimide amino N-oxide interfacial layers (Fig. 3b). Zhang et al. [40] developed a 3D hydrogenated amorphous silicon germanium radial junction PD on Al foil substrates, which allowed good signal monitoring at the wrist.
Oxygen Saturation (SpO2)
Oxygen saturation (SpO2) is the concentration of oxygen in blood. It represents the amount of oxygenated hemoglobin in blood as a percentage of the volume of all available bound hemoglobin. The saturation level of blood oxygen reflects health conditions and predicts diseases such as chronic obstructive pulmonary disease [41]. Generally, low SpO2 indicates insufficient oxygen supply, while high SpO2 could result in aging of cells in the body [42].
The main method of monitoring SpO2 is PPG, which is similar to the mechanism of heart rate monitoring by PPG. Usually, two different wavelengths of light are used in pulse oximeter. PD is capable of transducing changes in optical properties into electrical signals since oxygenated hemoglobin and non-oxygenated hemoglobin in vessels have different absorption rates for specific wavelengths of light [43,44,45]. Current skin-attachable oximeters have major problems with power consumption and size. Lee et al. [46] proposed an ultralow power reflective patch pulse oximeter (Fig. 4a). The average power of the proposed oximeter head decreased to 24 μW through a special design based on flexible organic light-emitting diodes (OLEDs) and organic photodiodes. The miniaturization of sensors further promoted the application of skin-attachable oximeter. Kim et al. [47] reported a sensing system capable of wirelessly capturing quantitative information about blood oxygen, heart rate, and heart rate variability (Fig. 4b). This system is battery-free and miniaturized. Han et al. [48] proposed an ambient light oximetry to address the impact of driving LEDs on the overall size of the sensor. The use of LEDs is avoided by combining spectral filters with OPD.
a Schematic of the organic pulse oximeter and the OLED, OPD structure used for the oximeter. Reprint with permission from Ref. [46]. Copyright 2018 American Association for the Advancement of Science. b Schematic illustration of the pulse oximeter sensing system. The sensor was attached to the nail for operation. Reprint with permission from Ref. [47]. Copyright 2017 John Wiley & Sons
Respiratory Rate
Respiratory diseases have become a serious health hazard in recent years due to atmospheric pollution and the outbreak of the COVID-19. During quarantine, healthcare workers and patients need to monitor their respiratory system status in real time. In the clinic, polysomnography (PSG) is frequently utilized for respiratory monitoring. The PSG monitoring instrument is usually connected to electrodes in multiple places on the body, which seriously affects daily activities of the patients. This shortcoming can be effectively overcome by skin-attachable respiratory rate sensor.
The first type of skin-attachable respiratory rate sensors is based on respiratory gas temperature and humidity. Taking temperature sensing as an example, the sensors are mainly attached to the nasal cavity or under the nose. The temperatures of the inhaled and exhaled gases are close to body temperature and room temperature, respectively. Respiratory rate can be accessed by measuring the temperature change of the inhaled and exhaled gases.
Metal oxides and nanomaterials are mainly used for respiratory humidity and temperature monitoring [49, 50]. Sufficient hydrophilic groups and high specific surface area enable graphene oxide (GO) to monitor humidity. Zhu et al. [49] fabricated diamine-based GO/mesoporous silica nanospheres. They introduced the decoration of diamino acid to improve the hydrophilic properties of graphene oxide, and used mesoporous structure of mesoporous silica nanospheres to tune the adsorption and desorption of water molecules, resulting in a more rapid response and higher sensitivity. There are also studies of integrated systems for long-term monitoring. Chen et al. [51] fabricated a skin-attachable sensor integrating flexible sensors and commercial chips for respiratory monitoring (Fig. 5a). These skin-like hybrid integrated circuits (SHICs) performed respiratory monitoring through detecting changes in the temperature of the breathing gas. SHICs can be used independently for signal processing and wireless transmission and extended with multiple sensors for functionality.
a Schematic illustrating the structure of the flexible sensor, with the SHIC bendable to attach the human facial skin for respiratory monitoring. Reprint with permission from Ref. [51]. Copyright 2020 John Wiley & Sons. b Schematic diagram of the SANES structure and enlarged view of Au-coated polyacrylonitrile and polyamide 66. Reprint with permission from Ref. [52]. Copyright 2021 John Wiley & Sons
The second method is to detect body posture changes and skin stretching during human breathing. Skin-attachable respiratory sensors typically exist in the form of patches or belts to detect changes in the body shape of chest and abdomen. Peng et al. [52] developed a TENG-based self-powered all-nanofiber e-skin (SANES) for real-time respiratory signal monitoring by electrospinning technique (Fig. 5b). SANES utilized Au-coated polyamides 66 and polyacrylonitrile nanofibers as top and bottom electrification layers of TENG, respectively. The porous structure between the nanofibers allowed higher sensitivity and power output of SANES.
Lung and Heart Sound
In the detection of cardiovascular and respiratory diseases, lung and heart sound signals can reflect the health status of relevant organs. Clinical trials have shown that abnormal heart sound is closely related to heart failure and other diseases [53]. Mechanical stethoscopes are mainly used to collect heart and lung sound signals, which are bulky and easily disturbed by the environment, making it unlikely to obtain scientific records and diagnoses in real-time monitoring and remote healthcare. Gupta et al. [54] combines the features of accelerometers and contact microphones to propose sensors that can simultaneously monitor respiratory rate, heart sound, and lung sound. Inspired by the human auditory system, Yan et al. [55] reported thermally woven fabrics with stretched composite piezoelectric fibers which enable auscultation (Fig. 6a). The fabric acted as a tympanic membrane, converting pressure waves into mechanical vibrations of the membrane. With a fiber optic transducer that output electrical signals similar to that of the cochlea, the skin-attachable fabric can effectively detect audible sounds with performance comparable to that of commercial microphones. For skin-attachable stethoscopes, noise between the sensor and skin contact can affect diagnostic results. Lee et al. [56] utilized different biocompatible adhesives to optimize motion artifacts and a wavelet denoising algorithm to improve SNR, allowing clinical applications of skin-attachable stethoscopes (Fig. 6b).
a Schematic illustrating the structure and principle of the fabric microphone. Twaron yarns and cotton yarn were arranged at right angles to mimic the structure of a tympanic membrane. Reprint with permission from Ref. [55]. Copyright 2022 Springer Nature. b Schematic illustrating the structure of the wearable stethoscope and optical image of the 180° bending stethoscope with finite element analysis results. Reprint with permission from Ref. [56]. Copyright 2022 American Association for the Advancement of Science
Chemical Sensor
In the biomedical applications of skin-attachable sensors, monitoring of chemical molecules is also crucial. The content and type of biomolecules in human sweat and saliva are closely related to health. However, traditional monitoring of chemical molecules is often invasive. Wearable skin-attachable sensor can more easily and quickly achieve non-invasive detection of biomolecules such as glucose and other biomarkers.
Glucose
In recent years, the incidence of diabetes has been increasing due to changes in people’s living habits. The number of people with diabetes has doubled in the last 20 years [57], making the disease a global human health challenge. There is a growing demand to monitor blood glucose in real time, especially for diabetic patients [58].
Traditional methods of blood glucose monitoring mostly use invasive blood tests, which can be physically and mentally taxing to patients who need to monitor their blood glucose frequently [59]. Since the glucose of body fluids such as sweat correlates with the concentration in the blood, skin-attachable sensors use non-invasive biological fluids such as sweat and saliva to detect glucose [60]. Skin-attachable glucose sensors minimize the pain and inconvenience of puncturing the skin, enabling non-invasive long-term glucose monitoring.
The main non-invasive mechanisms of measuring glucose are optical and electrochemical mechanisms, while the complexity of the readout and processing circuits limits the application of optical sensors [61]. Electrochemical methods have the longest history and mainly use enzyme-based methods to oxidize the substance to hydrogen peroxide, which is then catalyzed by nanomaterials. This method is highly specific and provides accurate detection results. Myndrul et al. [62] developed a skin-attachable enzyme-based glucose sensor based on ZnO tetrapods (TPs)/MXene. MXene nanoflake-decorated ZnO combines the best of the high relative surface area of ZnO TPs and the excellent conductivity of MXene nanoflakes, allowing the sensor to have a high sensitivity of 29 μAM−1 cm−2 and mechanical stability under 30% strain.
Although enzyme-based devices have high selectivity and sensitivity, the inherent instability of enzyme molecules under changes in pH, temperature, and humidity is unfavorable for wearable applications. The non-enzymatic glucose sensors are based on replacing biological enzymes with modified materials on electrodes to enhance electrocatalysis, which can overcome the drawbacks of enzyme molecules. The sensitivity of the non-enzymatic glucose sensor was controllably enhanced by plating Ni and Au on porous laser-induced graphene by Zhu et al. [63] (Fig. 7a). Zhang et al. [64] used electroless deposition process to prepare Cu nanoparticles anchored on laser-induced graphene (Cu NPs-LIG) (Fig. 7b). Benefiting from the matrix Cu, a highly sensitive non-enzymatic glucose sensor was proposed. The sensor’s response time of less than 0.5 s and low detection limit of 0.39 μM reveal the possibility of further applications for non-enzyme glucose sensors.
a Schematic diagram of the fabrication process and structure of the non-enzymatic glucose sensor. The laser-induced graphene electrode is covered with electroless plating Au and Ni. Reprint with permission from Ref. [63]. Copyright 2021 Elsevier. b Schematic diagram of the Cu NPs-LIG sensor fabrication process. Reprint with permission from Ref. [64]. Copyright 2019 Elsevier
Biomarker
Biomarkers are biochemical indicators that can mark possible changes in organ and tissue structures and functions. They can be used to determine the degree and stage of disease [65]. For example, cortisol could provide information on the biological state of the heart. Cancer biomarkers can be used to guide the treatment of tumor [66]. Non-invasive biofluids (e.g., sweat, saliva, urine and tears) remain the preferred route for detecting biomarkers. However, low levels of biomarkers in body fluids make detection very difficult, and the correspondence between the levels of biomarkers in non-invasive biofluids and in blood remains uncertain. The selectivity of receptors and the improvement of sensitivity could increase the potential for future applications of skin-attachable sensors.
Various kinds of biomarkers can be detected by skin-attachable sensors at present. Bandodkar et al. [67] developed a sensing platform by integrating microfluidics with an embedded colorimetric assay (Fig. 8a). Combining the advantages of electronic and microfluidic functions allowed the monitoring of pH, lactate, glucose, and chloride in sweat. Liu et al. [68] fabricated flexible In2O3 nanoribbon field effect transistor (FET) arrays to detect small molecule neurotransmitters. By attaching nucleic acid aptamers to In2O3 nanoribbons, detection limits of 10 fM for dopamine and serotonin can be achieved. Wang et al. [69] developed a flexible transistor biosensor array that utilized cortisol aptamers coupled to In2O3 FETs to achieve low detection limit cortisol monitoring (Fig. 8b). Cortisol levels could be recognized by the aptamer and converted into electrical signal on the FET.
a Schematic illustrating the structure of the skin-attachable microfluidic/electronic sensing system. Reprint with permission from Ref. [67]. Copyright 2019 American Association for the Advancement of Science. b Schematic diagram of the cortisol sensing principle and aptamer-FET-enabled biosensing smartwatch. Reprint with permission from Ref. [69]. Copyright 2022 American Association for the Advancement of Science
Multifunctional Sensor
Although diverse sensors detecting individual physiological parameters have been proposed, it is difficult to meet individual needs in real-world applications with a single function. Meanwhile, the diagnosis of disease generally requires consideration of different physiological indicators. For example, COVID-19 may exacerbate hyperglycemia along with abnormal pulmonary function [70]. Multifunctional sensors can detect multiple physiological indicators based on a same sensing mechanism, or integrating sensors with different mechanisms to achieve multifunctional health detection and disease treatment. Thus, multifunctional sensing can be implemented using layered structural designs of different sensors or single materials with different sensing functions.
Multifunctional sensors with a single structural design generally lead to a simple fabrication process but are not conducive to signal decoupling. Luo et al. [71] utilized MXene to fabricate a superhydrophobic and breathable textile-based multifunctional skin-attachable sensor to solve the problem of mechanical stability and environmental stability of the sensor under harsh environments (Fig. 9a). The textile exhibited excellent strain and temperature sensing properties by constructing a multiple core–shell structure. In contrast, the integration of multiple sensors can reduce signal interference and promote signal reliability but requires a complex structural design. Inspired by fingerprint, Zhu et al. [72] proposed a multifunctional sensor for pressure and temperature sensing using a region-partition strategy. Interferences between sensing units were effectively reduced by the strain isolation structure. Sempionatto et al. [73] combined ultrasound and electrochemical sensors to develop a skin-attachable patch that could simultaneously monitor blood pressure(BP) and heart rate(HR) as well as multiple biomarkers (Fig. 9b). BP and HR were monitored using ultrasound sensors, and biomarker levels were measured by electrochemical sensors. The integration of custom piezoelectric zirconate titanate ultrasonic sensors and printed polymer composites through a solvent-soldering process overcame the difficulties of integrating sensors with different sensing modalities.
a Schematic diagram of the fabrication process and structure of MXene-based textile. The textile can attach to the skin of the joints for motion and temperature monitoring. Reprint with permission from Ref. [71]. Copyright 2020 Elsevier. b Schematic of the skin-attachable patch, including the acoustic and electrochemical components of the sensor. The skin-attachable patch can access BP, HR and electrochemical signals to monitor a person's physiological status. Reprint with permission from Ref. [73]. Copyright 2021 Springer Nature
Conclusion and Outlook
In this review, we summarize the mechanisms and recent advances of skin-attachable sensors for biomedical and healthcare applications. The current skin-attachable sensors for biomedical applications are mainly physical and chemical sensors, and they can non-invasively monitor respiratory rate, blood oxygen saturation, glucose, and biomarkers. Conformal fit of the sensor to the skin improves wearing comfort and data accuracy. Meanwhile, multifunctional skin-attachable sensor that monitors multiple indicators is an important direction for future development.
Skin-attachable sensor is an interdisciplinary and fast-growing subject under increasing demand for household healthcare and the outbreak of the pandemic in recent years. In the future, skin-attachable sensor still has some important development directions.
-
(1)
Currently, the types of biomolecules measured are limited. Multifunctional and integrated sensors are still the trend. With the development of research, skin-attachable sensor will integrate more different types of sensors, enabling uninterrupted long-term and diverse monitoring of physiological indicators.
-
(2)
Few skin-attachable sensors are currently commercially available. Performance testing in experimental environment and the complexity of the process hinder the practical applications of the sensors. Further research is needed to improve the tolerance of skin-attachable sensors to the external environment and process compatibility to increase the potential for large-scale applications.
-
(3)
Skin-attachable sensors can continuously monitor biomedical characteristics, which will obtain a large amount of data. How to process and analyze these data to achieve practical applications in medical care is still a major problem. The combination of big data, artificial intelligence, and advanced processing algorithms can improve the accuracy of data and provide a comprehensive analysis of an individual’s health status.
References
Z. Shahid, R. Kalayanamitra, B. McClafferty, D. Kepko, D. Ramgobin, R. Patel, C.S. Aggarwal, R. Vunnam, N. Sahu, D. Bhatt, K. Jones, R. Golamari, R. Jain, COVID-19 and older adults: what we know. J. Am. Geriatr. Soc. 68, 926–929 (2020). https://doi.org/10.1111/jgs.16472
X. Ding, D. Clifton, N. Ji, N.H. Lovell, P. Bonato, W. Chen, X. Yu, Z. Xue, T. Xiang, X. Long, K. Xu, X. Jiang, Q. Wang, B. Yin, G. Feng, Y.-T. Zhang, Wearable sensing and telehealth technology with potential applications in the coronavirus pandemic. IEEE Rev. Biomed. Eng. 14, 48–70 (2021). https://doi.org/10.1109/RBME.2020.2992838
H.C. Ates, A.K. Yetisen, F. Güder, C. Dincer, Wearable devices for the detection of COVID-19. Nat. Electron. 4, 13–14 (2021). https://doi.org/10.1038/s41928-020-00533-1
A. Channa, N. Popescu, J. Skibinska, R. Burget, The rise of wearable devices during the COVID-19 pandemic: a systematic review. Sensors 21, 5787 (2021). https://doi.org/10.3390/s21175787
H.-R. Lim, H.S. Kim, R. Qazi, Y.-T. Kwon, J.-W. Jeong, W.-H. Yeo, Advanced soft materials, sensor integrations, and applications of wearable flexible hybrid electronics in healthcare, energy, and environment. Adv. Mater. 32, 1901924 (2020). https://doi.org/10.1002/adma.201901924
J.C. Yang, J. Mun, S.Y. Kwon, S. Park, Z. Bao, S. Park, Electronic skin: recent progress and future prospects for skin-attachable devices for health monitoring, robotics, and prosthetics. Adv. Mater. 31, 1904765 (2019). https://doi.org/10.1002/adma.201904765
B.H. Kang, K. Park, M. Hambsch, S. Hong, H.T. Kim, D.H. Choi, J.H. Lee, S. Kim, H.J. Kim, Skin-conformable photoplethysmogram sensors for energy-efficient always-on cardiovascular monitoring systems. Nano Energy 92, 106773 (2022). https://doi.org/10.1016/j.nanoen.2021.106773
H. Jin, J. Yu, S. Lin, S. Gao, H. Yang, H. Haick, C. Hua, S. Deng, T. Yang, Y. Liu, W. Shen, X. Zhang, X. Zhang, S. Shan, T. Ren, L. Wang, W. Cheung, W. Kam, J. Miao, D. Chen, D. Cui, Nanosensor-based flexible electronic assisted with light fidelity communicating technology for volatolomics-based telemedicine. ACS Nano 14, 15517–15532 (2020). https://doi.org/10.1021/acsnano.0c06137
J. Yu, X. Hou, J. He, M. Cui, C. Wang, W. Geng, J. Mu, B. Han, X. Chou, Ultra-flexible and high-sensitive triboelectric nanogenerator as electronic skin for self-powered human physiological signal monitoring. Nano Energy 69, 104437 (2020). https://doi.org/10.1016/j.nanoen.2019.104437
M. Du, Y. Cao, X. Qu, J. Xue, W. Zhang, X. Pu, B. Shi, Z. Li, Hybrid nanogenerator for biomechanical energy harvesting, motion state detection, and pulse sensing. Adv. Mater. Technol. (2022). https://doi.org/10.1002/admt.202101332
K.S. Chun, Y.J. Kang, J.Y. Lee, M. Nguyen, B. Lee, R. Lee, H.H. Jo, E. Allen, H. Chen, J. Kim, L. Yu, X. Ni, K. Lee, H. Jeong, J. Lee, Y. Park, H.U. Chung, A.W. Li, P.A. Lio, A.F. Yang, A.B. Fishbein, A.S. Paller, J.A. Rogers, S. Xu, A skin-conformable wireless sensor to objectively quantify symptoms of pruritus. Sci. Adv. 7, eabf9405 (2021). https://doi.org/10.1126/sciadv.abf9405
T. Ray, J. Choi, J. Reeder, S.P. Lee, A.J. Aranyosi, R. Ghaffari, J.A. Rogers, Soft, skin-interfaced wearable systems for sports science and analytics. Curr. Opin. Biomed. Eng. 9, 47–56 (2019). https://doi.org/10.1016/j.cobme.2019.01.003
P. Picerno, M. Iosa, C. D’Souza, M.G. Benedetti, S. Paolucci, G. Morone, Wearable inertial sensors for human movement analysis: a five-year update. Expert Rev. Med. Dev. 18, 79–94 (2021). https://doi.org/10.1080/17434440.2021.1988849
R. Sun, J.J. Sosnoff, Novel sensing technology in fall risk assessment in older adults: a systematic review. BMC Geriatr. 18, 14 (2018). https://doi.org/10.1186/s12877-018-0706-6
S. Chen, J. Qi, S. Fan, Z. Qiao, J.C. Yeo, C.T. Lim, Flexible wearable sensors for cardiovascular health monitoring. Adv. Healthc. Mater. 10, 2100116 (2021). https://doi.org/10.1002/adhm.202100116
M. Ringeval, G. Wagner, J. Denford, G. Pare, S. Kitsiou, Fitbit-based interventions for healthy lifestyle outcomes: systematic review and meta-analysis. J. Med. Internet Res. 22, e23954 (2020). https://doi.org/10.2196/23954
C. Tuncay, M.C. Ergoren, A systematic review of precision nutrition and Mediterranean Diet: a personalized nutrition approaches for prevention and management of obesity related disorders. Clin. Nutr. ESPEN 38, 61–64 (2020). https://doi.org/10.1016/j.clnesp.2020.04.005
C. Ma, M.-G. Ma, C. Si, X.-X. Ji, P. Wan, Flexible MXene-based composites for wearable devices. Adv. Funct. Mater. 31, 2009524 (2021). https://doi.org/10.1002/adfm.202009524
Q. Zhang, X. Liu, L. Duan, G. Gao, Ultra-stretchable wearable strain sensors based on skin-inspired adhesive, tough and conductive hydrogels. Chem. Eng. J. 365, 10–19 (2019). https://doi.org/10.1016/j.cej.2019.02.014
Y. Qiao, X. Li, J. Jian, Q. Wu, Y. Wei, H. Shuai, T. Hirtz, Y. Zhi, G. Deng, Y. Wang, G. Gou, J. Xu, T. Cui, H. Tian, Y. Yang, T.-L. Ren, Substrate-free multilayer graphene electronic skin for intelligent diagnosis. ACS Appl. Mater. Interfaces 12, 49945–49956 (2020). https://doi.org/10.1021/acsami.0c12440
Y. Song, D. Mukasa, H. Zhang, W. Gao, Self-powered wearable biosensors. Acc. Mater. Res. 2, 184–197 (2021). https://doi.org/10.1021/accountsmr.1c00002
X. Zeng, R. Peng, Z. Fan, Y. Lin, Self-powered and wearable biosensors for healthcare. Mater. Today Energy 23, 100900 (2022). https://doi.org/10.1016/j.mtener.2021.100900
J. Huang, J. Zeng, X. Zhang, G. Guo, R. Liu, Z. Yan, Y. Yin, Fatigue resistant aerogel/hydrogel nanostructured hybrid for highly sensitive and ultrabroad pressure sensing. Small 18, 2104706 (2022). https://doi.org/10.1002/smll.202104706
X. Chen, H. Liu, Y. Zheng, Y. Zhai, X. Liu, C. Liu, L. Mi, Z. Guo, C. Shen, Highly compressible and robust polyimide/carbon nanotube composite aerogel for high-performance wearable pressure sensor. ACS Appl. Mater. Interfaces 11, 42594–42606 (2019). https://doi.org/10.1021/acsami.9b14688
Z. He, G. Zhou, J.-H. Byun, S.-K. Lee, M.-K. Um, B. Park, T. Kim, S.B. Lee, T.-W. Chou, Highly stretchable multi-walled carbon nanotube/thermoplastic polyurethane composite fibers for ultrasensitive, wearable strain sensors. Nanoscale 11, 5884–5890 (2019). https://doi.org/10.1039/c9nr01005j
K. Dong, X. Peng, J. An, A.C. Wang, J. Luo, B. Sun, J. Wang, Z.L. Wang, Shape adaptable and highly resilient 3D braided triboelectric nanogenerators as e-textiles for power and sensing. Nat. Commun. (2020). https://doi.org/10.1038/s41467-020-16642-6
X. Zhang, Z. Li, W. Du, Y. Zhao, W. Wang, L. Pang, L. Chen, A. Yu, J. Zhai, Self-powered triboelectric-mechanoluminescent electronic skin for detecting and differentiating multiple mechanical stimuli. Nano Energy 96, 107115 (2022). https://doi.org/10.1016/j.nanoen.2022.107115
L. Dong, M. Wang, J. Wu, C. Zhu, J. Shi, H. Morikawa, Stretchable, adhesive, self-healable, and conductive hydrogel-based deformable triboelectric nanogenerator for energy harvesting and human motion sensing. ACS Appl. Mater. Interfaces 14, 9126–9137 (2022). https://doi.org/10.1021/acsami.1c23176
M. Mariello, L. Fachechi, F. Guido, M. De Vittorio, Conformal, ultra-thin skin-contact-actuated hybrid piezo/triboelectric wearable sensor based on AlN and parylene-encapsulated elastomeric blend. Adv. Funct. Mater. 31, 2101047 (2021). https://doi.org/10.1002/adfm.202101047
H. Shao, J. Fang, H. Wang, L. Dai, T. Lin, Polymer-metal Schottky contact with direct-current outputs. Adv. Mater. 28, 1461–1466 (2016). https://doi.org/10.1002/adma.201504778
Y. Yang, G. Zhou, D. Ye, Z. Bai, Z. Deng, J. Xu, A self-powered laminated fabric sensor for human motion detection and heart-rate monitoring based on PPy/Al Schottky contact. J. Sandwich Struct. Mater. 24, 503–516 (2022). https://doi.org/10.1177/10996362211021889
R. Yang, M. Benner, Z. Guo, C. Zhou, J. Liu, High-Performance flexible Schottky DC generator via metal/conducting polymer sliding contacts. Adv. Funct. Mater. 31, 2103132 (2021). https://doi.org/10.1002/adfm.202103132
R.J. Myerburg, M.J. Junttila, Sudden cardiac death caused by coronary heart disease. Circulation 125, 1043–1052 (2012). https://doi.org/10.1161/CIRCULATIONAHA.111.023846
V. Patel, M. Danish, C. Monteleone, All roads “lead” to anaphylaxis: hypersensitivity to electrocardiogram leads. Ann. Allergy Asthma Immunol. 121, S78–S79 (2018). https://doi.org/10.1016/j.anai.2018.09.258
M.O.G. Nayeem, S. Lee, H. Jin, N. Matsuhisa, H. Jinno, A. Miyamoto, T. Yokota, T. Someya, All-nanofiber–based, ultrasensitive, gas-permeable mechanoacoustic sensors for continuous long-term heart monitoring. Proc. Natl. Acad. Sci. USA 117, 7063–7070 (2020). https://doi.org/10.1073/pnas.1920911117
Y. Wang, X. Zhong, W. Wang, D. Yu, Flexible cellulose/polyvinyl alcohol/PEDOT:PSS electrodes for ECG monitoring. Cellulose 28, 4913–4926 (2021). https://doi.org/10.1007/s10570-021-03818-6
P. Won, J.J. Park, T. Lee, I. Ha, S. Han, M. Choi, J. Lee, S. Hong, K.-J. Cho, S.H. Ko, Stretchable and transparent Kirigami conductor of nanowire percolation network for electronic skin applications. Nano Lett. 19, 6087–6096 (2019). https://doi.org/10.1021/acs.nanolett.9b02014
B. Bent, B.A. Goldstein, W.A. Kibbe, J.P. Dunn, Investigating sources of inaccuracy in wearable optical heart rate sensors. Npj Digit. Med. 3, 18 (2020). https://doi.org/10.1038/s41746-020-0226-6
J. Simoes, T. Dong, Z. Yang, Non-fullerene acceptor organic photodetector for skin-conformable photoplethysmography applications. Adv. Mater. Interfaces 9, 2101897 (2022). https://doi.org/10.1002/admi.202101897
S. Zhang, T. Zhang, Z. Liu, J. Wang, J. Xu, K. Chen, L. Yu, Flexible and robust 3D a-SiGe radial junction near-infrared photodetectors for rapid sphygmic signal monitoring. Adv. Funct. Mater. 32, 2107040 (2022). https://doi.org/10.1002/adfm.202107040
J. Buekers, J. Theunis, P.D. Boever, A.W. Vaes, M. Koopman, E.V. Janssen, E.F. Wouters, M.A. Spruit, J.-M. Aerts, Wearable finger pulse oximetry for continuous oxygen saturation measurements during daily home routines of patients with chronic obstructive pulmonary disease (COPD) over one week: observational study. JMIR Mhealth Uhealth 7, e12866 (2019). https://doi.org/10.2196/12866
R. Ortega, C.J. Hansen, K. Elterman, A. Woo, Videos in clinical medicine. Pulse oximetry. N. Engl. J. Med. 364, e33 (2011). https://doi.org/10.1056/nejmvcm0904262
Y. Wang, H. Haick, S. Guo, C. Wang, S. Lee, T. Yokota, T. Someya, Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. (2018). https://doi.org/10.1039/d2cs00207h
M.S. Roy, R. Gupta, J.K. Chandra, K. Das Sharma, A. Talukdar, Improving photoplethysmographic measurements under motion artifacts using artificial neural network for personal healthcare. IEEE Trans. Instrum. Meas. 67, 2820–2829 (2018). https://doi.org/10.1109/TIM.2018.2829488
F. Rundo, S. Conoci, A. Ortis, S. Battiato, An advanced bio-inspired photoplethysmography (PPG) and ECG pattern recognition system for medical assessment. Sensors 18, 405 (2018). https://doi.org/10.3390/s18020405
H. Lee, E. Kim, Y. Lee, H. Kim, J. Lee, M. Kim, H.-J. Yoo, S. Yoo, Toward all-day wearable health monitoring: an ultralow-power, reflective organic pulse oximetry sensing patch. Sci. Adv. 4, eaas9530 (2018). https://doi.org/10.1126/sciadv.aas9530
J. Kim, P. Gutruf, A.M. Chiarelli, S.Y. Heo, K. Cho, Z. Xie, A. Banks, S. Han, K.-I. Jang, J.W. Lee, K.-T. Lee, X. Feng, Y. Huang, M. Fabiani, G. Gratton, U. Paik, J.A. Rogers, Miniaturized battery-free wireless systems for wearable pulse oximetry. Adv. Funct. Mater. 27, 1604373 (2017). https://doi.org/10.1002/adfm.201604373
D. Han, Y. Khan, J. Ting, J. Zhu, C. Combe, A. Wadsworth, I. McCulloch, A.C. Arias, Pulse oximetry using organic optoelectronics under ambient light. Adv. Mater. Technol. 5, 1901122 (2020). https://doi.org/10.1002/admt.201901122
J. Zhu, N. Zhang, Y. Yin, B. Xu, W. Zhang, C. Wang, High-sensitivity and low-hysteresis GO-NH2/mesoporous SiO2 nanosphere-fabric-based humidity sensor for respiratory monitoring and noncontact sensing. Adv. Mater. Interfaces 9, 2101498 (2022). https://doi.org/10.1002/admi.202101498
S. Zou, L.-Q. Tao, G. Wang, C. Zhu, Z. Peng, H. Sun, Y. Li, Y. Wei, T.-L. Ren, Humidity-based human-machine interaction system for healthcare applications. ACS Appl. Mater. Interfaces 14, 12606–12616 (2022). https://doi.org/10.1021/acsami.1c23725
Y. Chen, F. Liu, B. Lu, Y. Zhang, X. Feng, Skin-like hybrid integrated circuits conformal to face for continuous respiratory monitoring. Adv. Electron. Mater. 6, 2000145 (2020). https://doi.org/10.1002/aelm.202000145
X. Peng, K. Dong, C. Ning, R. Cheng, J. Yi, Y. Zhang, F. Sheng, Z. Wu, Z.L. Wang, All-nanofiber self-powered skin-interfaced real-time respiratory monitoring system for obstructive sleep apnea-hypopnea syndrome diagnosing. Adv. Funct. Mater. 31, 2103559 (2021). https://doi.org/10.1002/adfm.202103559
M.H. Drazner, J.E. Rame, L.W. Stevenson, D.L. Dries, Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N. Engl. J. Med. 345, 574–581 (2001). https://doi.org/10.1056/NEJMoa010641
P. Gupta, M.J. Moghimi, Y. Jeong, D. Gupta, O.T. Inan, F. Ayazi, Precision wearable accelerometer contact microphones for longitudinal monitoring of mechano-acoustic cardiopulmonary signals. Npj Digit. Med. 3, 19 (2020). https://doi.org/10.1038/s41746-020-0225-7
W. Yan, G. Noel, G. Loke, E. Meiklejohn, T. Khudiyev, J. Marion, G. Rui, J. Lin, J. Cherston, A. Sahasrabudhe, J. Wilbert, I. Wicaksono, R.W. Hoyt, A. Missakian, L. Zhu, C. Ma, J. Joannopoulos, Y. Fink, Single fibre enables acoustic fabrics via nanometre-scale vibrations. Nature 603, 616–623 (2022). https://doi.org/10.1038/s41586-022-04476-9
S.H. Lee, Y.-S. Kim, M.-K. Yeo, M. Mahmood, N. Zavanelli, C. Chung, J.Y. Heo, Y. Kim, S.-S. Jung, W.-H. Yeo, Fully portable continuous real-time auscultation with a soft wearable stethoscope designed for automated disease diagnosis. Sci. Adv. 8, eabo5867 (2022). https://doi.org/10.1126/sciadv.abo5867
P.Z. Zimmet, D.J. Magliano, W.H. Herman, J.E. Shaw, Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2, 56–64 (2014). https://doi.org/10.1016/S2213-8587(13)70112-8
H. Teymourian, A. Barfidokht, J. Wang, Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem. Soc. Rev. 49, 7671–7709 (2020). https://doi.org/10.1039/d0cs00304b
H. Lee, Y.J. Hong, S. Baik, T. Hyeon, D.-H. Kim, Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthc. Mater. 7, 1701150 (2018). https://doi.org/10.1002/adhm.201701150
Y. Chen, S. Lu, S. Zhang, Y. Li, Z. Qu, Y. Chen, B. Lu, X. Wang, X. Feng, Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 3, e1701629 (2017). https://doi.org/10.1126/sciadv.1701629
L. Tang, S.J. Chang, C.-J. Chen, J.-T. Liu, Non-invasive blood glucose monitoring technology: a review. Sensors 20, 6925 (2020). https://doi.org/10.3390/s20236925
V. Myndrul, E. Coy, N. Babayevska, V. Zahorodna, V. Balitskyi, I. Baginskiy, O. Gogotsi, M. Bechelany, M.T. Giardi, I. Iatsunskyi, MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 207, 114141 (2022). https://doi.org/10.1016/j.bios.2022.114141
J. Zhu, S. Liu, Z. Hu, X. Zhang, N. Yi, K. Tang, M.G. Dexheimer, X. Lian, Q. Wang, J. Yang, J. Gray, H. Cheng, Laser-induced graphene non-enzymatic glucose sensors for on-body measurements. Biosens. Bioelectron. 193, 113606 (2021). https://doi.org/10.1016/j.bios.2021.113606
Y. Zhang, N. Li, Y. Xiang, D. Wang, P. Zhang, Y. Wang, S. Lu, R. Xu, J. Zhao, A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 156, 506–513 (2020). https://doi.org/10.1016/j.carbon.2019.10.006
R.M. Califf, Biomarker definitions and their applications. Exp. Biol. Med. 243, 213–221 (2018). https://doi.org/10.1177/1535370217750088
C.L. Sawyers, The cancer biomarker problem. Nature 452, 548–552 (2008). https://doi.org/10.1038/nature06913
A.J. Bandodkar, P. Gutruf, J. Choi, K. Lee, Y. Sekine, J.T. Reeder, W.J. Jeang, A.J. Aranyosi, S.P. Lee, J.B. Model, R. Ghaffari, C.-J. Su, J.P. Leshock, T. Ray, A. Verrillo, K. Thomas, V. Krishnamurthi, S. Han, J. Kim, S. Krishnan, T. Hang, J.A. Rogers, Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019). https://doi.org/10.1126/sciadv.aav3294
Q. Liu, C. Zhao, M. Chen, Y. Liu, Z. Zhao, F. Wu, Z. Li, P.S. Weiss, A.M. Andrews, C. Zhou, Flexible multiplexed In2O3 nanoribbon aptamer-field-effect transistors for biosensing. iScience 23, 101469 (2020). https://doi.org/10.1016/j.isci.2020.101469
B. Wang, C. Zhao, Z. Wang, K.-A. Yang, X. Cheng, W. Liu, W. Yu, S. Lin, Y. Zhao, K.M. Cheung, H. Lin, H. Hojaiji, P.S. Weiss, M.N. Stojanovic, A.J. Tomiyama, A.M. Andrews, S. Emaminejad, Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 8, eabk0967 (2022). https://doi.org/10.1126/sciadv.abk0967
P.-H. Tsai, W.-Y. Lai, Y.-Y. Lin, Y.-H. Luo, Y.-T. Lin, H.-K. Chen, Y.-M. Chen, Y.-C. Lai, L.-C. Kuo, S.-D. Chen, K.-J. Chang, C.-H. Liu, S.-C. Chang, F.-D. Wang, Y.-P. Yang, Clinical manifestation and disease progression in COVID-19 infection. J. Chin. Med. Assoc. 84, 3–8 (2021). https://doi.org/10.1097/JCMA.0000000000000463
J. Luo, S. Gao, H. Luo, L. Wang, X. Huang, Z. Guo, X. Lai, L. Lin, R.K.Y. Li, J. Gao, Superhydrophobic and breathable smart MXene-based textile for multifunctional wearable sensing electronics. Chem. Eng. J. 406, 126898 (2021). https://doi.org/10.1016/j.cej.2020.126898
L. Zhu, Y. Wang, D. Mei, W. Ding, C. Jiang, Y. Lu, Fully elastomeric fingerprint-shaped electronic skin based on tunable patterned graphene/silver nanocomposites. ACS Appl. Mater. Interfaces 12, 31725–31737 (2020). https://doi.org/10.1021/acsami.0c09653
J.R. Sempionatto, M. Lin, L. Yin, E. De la Paz, K. Pei, T. Sonsaard, A.N. de Loyola Silva, A.A. Khorshed, F. Zhang, N. Tostado, S. Xu, J. Wang, An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021). https://doi.org/10.1038/s41551-021-00685-1
Acknowledgements
This work was supported by the National Key Research and Development program of China under Grant No. 2021YFA1401103; The National Science Fund for Distinguished Young Scientists under Grant No. 61825403; The National Natural Science Foundation of China under Grants No. 61921005 and 61674078; and the Key Project supported by Medical Science and Technology Development foundation, Nanjing Department of Health under Grant (ZKX21055).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hua, J., Li, J., Jiang, Y. et al. Skin-Attachable Sensors for Biomedical Applications. Biomedical Materials & Devices 1, 256–268 (2023). https://doi.org/10.1007/s44174-022-00018-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-022-00018-z