Abstract
The traditional approach for the beneficiation of South African siliceous sedimentary phosphate ores has been reverse cationic flotation. The robustness of this approach, however, has been a concern due to its sensitivity to parameters such as slimes content, headgrade (P2O5 and SiO2 content), reagent dosages and conditioning times. The phosphate mineralisation for the ore body under investigation is predominantly carbonate fluorapatite composed of mainly quartz and apatite and minor amounts of chlorite, ilmenite and carbonates. Beneficiation of low grade sedimentary ores containing calcareous impurities, such as calcite and/or dolomite in addition to siliceous gangue, is required to meet the increasing demand; therefore, much research has been carried out to find an alternative technique to beneficiate this material. This paper investigates traditional flotation approaches versus the currently adopted ‘Crago Process’. Comparative results are presented in terms of upgrade potential for the production of concentrate rock for downstream applications. The novelty of the ‘Crago Process’ lies in its double stage approach, i.e. direct fatty acid flotation followed by reverse cationic amine flotation. The Crago process was able to achieve higher upgrades reporting at 32.1% P2O5 compared to conventional cationic flotation only achieving grades of 18.5% P2O5. Furthermore, the recovery gain was 3%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the growth in the global population, phosphate production has been one of the fastest growing areas in industrial minerals. Phosphate rock is essential for global food security with approximately 90% used in the production of fertilisers. South Africa has various types of phosphate deposits including commercially exploited igneous deposits, sedimentary phosphate deposits and marine sedimentary phosphorites. Sedimentary rocks being more abundant and hence the focus of the study generally contain higher-grade ore but recovery and final concentration of phosphorus pentoxide (P2O5) are lower than with igneous ore. The most important distinction between the two is the presence of contaminants.
With the rapid depletion of high quality reserves, this research focused on South Africa’s primary sedimentary phosphate ore body with a view of optimising process operations in line with market specifications of > 31% P2O5 and < 3% combined minor elements (Al2O3 + Fe2O3 + MgO). For ease of reporting, the combined oxides are referred to as X2O3. The research investigated the beneficiation potential of the phosphate ore by traditional reverse cationic flotation versus the plant adopted Crago process.
2 South Africa’s Primary Sedimentary Phosphate Deposit
Kropz Elandsfontein (Pty) Limited, South Africa’s second largest phosphate producer operates the Elandsfontein mine. This mine contains a significant resource of phosphate and is situated on the West Coast of South Africa, approximately 95 km northwest of Cape Town, within the Saldanha Bay Municipality.
The Council of Geoscience, formerly the Geological Survey of South Africa, regards the Elandsfontein deposit as the largest sedimentary deposit in South Africa [1]. The phosphate mineralisation is contained in the Varswater Formation, with the phosphate occurring in two forms, namely phosphatised shell fragments and phosphorite pellets. The Elandsfontein sedimentary phosphate deposit has a projected initial life of mine of about 14 years and can deliver one million tonnes a year of phosphate rock concentrate at a steady state. The resource was defined by Snowden and SRK in October 2018 on a total (gross) basis with 74% attributable to the company as reflected in Table 1. No further geological drilling was conducted in 2020 to re-affirm the values presented [2].
The Elandsfontein plant produces > 1Mtpa of rock phosphate at a target product grade of > 31% P2O5. Based on historical work, it was found that by increasing the final product grade, recovery was significantly compromised.
3 Case Study on a South African Sedimentary Deposit
3.1 Method
3.1.1 Test Samples
Two phosphorite sandy samples, namely F1 and F2 weighing 85 kg and 83 kg respectively, were supplied for research purposes. Approximately 20 kg was removed upfront from each sample and retained. The remainder was combined according to the mining ratio of 45:55 for F1 and F2 respectively to provide a representative composite sample.
The composite sample weighed approximately 60 kg. Representative sub-samples were removed for headgrade analysis, size-by-assay analysis and mineralogical characterisation prior to downstream process optimisation tests.
3.1.2 Mineralogical Assessment
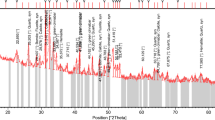
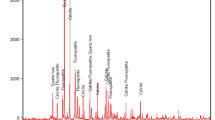
The feed composite was submitted for mineralogical investigation with the aim of characterising the apatite mineralisation in terms of mineral chemistry and impurities as well as liberation, association and grain size distribution using a combination of quantitative X-ray diffraction (XRD), and automated SEM (autoSEM) analysis.
3.1.3 Bulk Milling and Screening
Based on mineralogical evaluation, approximately 40 kg of the composite feed was rod milled to 100% passing 425 μm. This grind size was determined as producing sufficiently liberated apatite from gangue (> 80% liberation). Prior to bulk milling, 3 × 1 kg batches were milled at an energy input of 7.5 kWh/t as per indication from grindmill testing. The aim was to determine reproducibility of results prior to bulk milling.
The bulk milled sample was thereafter subjected to screening at 212 μm and 25 μm to generate − 425 + 212 μm and − 212 + 25 μm sub-fractions for ensuing flotation tests. The − 25 μm slimes fraction was chemically assayed for completion of overall mass balances.
3.1.4 Reverse Cationic Flotation
Laboratory flotation testwork was conducted on the composited feed at a grind of 100% passing 425 μm. The sample was slurried and slimes rudimentarily decanted, which would otherwise affect float efficiency.
Reverse cationic flotation was carried out based purely on visual observations and using lilaflot collector as was previously tested for alternative sedimentary phosphate ores. Lilaflot D817M is an etherdiamine suitable for removal of silicates. It should be noted that reagent dosage optimisation was not considered for these tests as a visual product was targeted. A sub-sample of 1 kg was subjected to a 2.5 L flotation cell using the Denver flotation machine agitated at 1200 rpm air flowrate.
The testwork conditions are represented in Table 2 with a schematic of the flowsheet represented by Fig. 1.
3.1.5 ‘Crago Process’
Processing of Elandsfontein material currently follows the ‘Crago’ process. The Crago process for the flotation of sedimentary phosphate was patented in 1942. This direct-reverse flotation process consists of a fatty acid rougher stage followed by deoiling and an amine cleaner flotation stage which is the standard for phosphate flotation in Florida and North Carolina [3]. Due to the upfront addition of fatty acid, an intermediate acid scrubbing stage using sulphuric acid followed by washing is required prior to reverse flotation using an amine collector. This is an important step to clean the particle surfaces prior to addition of amine for reverse flotation. Figure 2 is a schematic representation of the Crago process.
In the Crago process, the crushed and sized feed is subjected to a rougher flotation stage. Conditioning is undertaken by adding fatty acid and diesel to maintain a pH of around 9–9.5 for about 3 min at 30% solids. The phosphate is then directly floated with the rougher concentrate being subjected to acid scrubbing using sulphuric acid and washing to remove the reagents from the phosphate surfaces. Reverse flotation is the next step in the process using an amine collector. It should be noted that a considerable amount of the silicates/carbonates are removed in the rougher stage upfront. The reverse flotation stage removes residual silica at a natural pH. In the conventional Crago process, flotation is carried out by ‘Double Float’, that is, the feed is floated twice using fatty acid and then with amine [3, 4].
The milled feed was screened at 212 μm, with intermediate fractions subjected to the Crago process. Optimised test conditions are represented below for each fraction in Tables 3 and 4. A schematic of the test process adopted is shown in Fig. 3.
4 Results and Discussion
4.1 Ore Characterisation: Size by Assay Analysis
The headgrade of the composited feed reported at 8.0% P2O5, 74.5% SiO2, 1.2 ppm Cd and 1.6% X2O3. Figure 4 represents the composite feed particle size distribution (PSD) prepared for testwork purposes as well as mineralogical evaluation showing reproducibility in sizing. Figure 5 represents the discrete mass distribution and elemental deportment by size.
In terms of mass distribution, the majority of the feed lies between the intermediate size class of − 212 + 125 μm at approximately 51%. The composite feed indicates that there is only about 5% of naturally occurring slimes (− 38 μm) at approximately 15% P2O5 deportment. This fraction, however, contains a higher contained deleterious element content of approximately 12% X2O3.
4.2 Mineralogical Assessment
Phosphate is present as carbonate fluorapatite. The apatite in the sample is typically well liberated with limited association (Fig. 6). Apatite poorly liberated occurs associated with quartz, commonly as rims along quartz grains or as host to quartz inclusions (Fig. 7) occurring in several forms including pristine apatite, silica-rich apatite (containing quartz inclusions) and silica-poor apatite (containing abundant cracks).
The apatite is well liberated, particularly in the finer size fractions (− 150 + 125 µm and − 125 µm). The coarsest size fraction (+ 850 µm) is the only fraction displaying poorly liberated apatite; however, this represents a relatively small contribution to the sample (< 5%).
It is worth noting that even pristine apatite contains micro-inclusions of Al, Fe and S.
4.3 Bulk Milling and Screening
Milling of the composited feed was targeted at 100% passing 425 µm for downstream testing. Sub-samples were subjected to comparative ball and rod milling tests (Fig. 8) with the bulk sample undergoing rod milling in an attempt to reduce the fines generated.
Rod milling was able to achieve the target grind producing a coarser product compared to ball milling. The grind distribution obtained for 3 and 4 min being virtually the same. The size distribution analysis for the rod milled bulk sample is represented in Table 5.
Rod milling was able to achieve double the mass percentage reporting to the coarser size fraction (19.42% as opposed to 9.08%) whilst maintaining target grind. This mass distribution is more in-line with current plant setup and hence, ensuing tests were conducted on rod milled product samples.
4.4 Reverse Cationic Flotation
Table 6 presents the results for reverse cationic flotation using an amine lilaflot reagent.
Reverse flotation achieved a P2O5 upgrade ratio of 2.2 at 59.0% recovery. The P2O5 upgrade, however, does not meet target specifications of > 31% P2O5. Based on visual observations and flotation performance, efficient desliming needs to be implemented to remove slimes which ultimately results in high P2O5 losses. The grade of X2O3 averaged 1.8% in the phosphate product (RT), well below the < 3% specification. The rejection of silica was very good at 83.6% to the final product; however, contained Si content is still high within the product at 45.2%. Further process optimisation was therefore required and hence tests to investigate the current methodology adopted at the plant and consider ways of improving performance and final product grade and recovery were pursued.
4.5 ‘Crago’ Flotation
After testing multiple reagent dosages, optimised results were obtained as presented below. A Crago direct-reverse test was pursued on the coarse (−425+212 μm) and fines (−212+25 μm) fractions in an attempt to improve the overall P2O5 recovery and reduce reagent consumption. Table 7 represents the overall mass balance for the Crago coarse and fines process.
The combined effect of the direct-reverse flowsheet shows that P2O5 can be upgraded to a final concentrate of 32.1% P2O5 at an overall recovery of 61.0% higher than the recovery achieved for the reverse cationic float test reporting at 59.0%. The contaminant specification was achieved at 2.8% X2O3.
5 Conclusion
The headgrade of the composited feed reported at 8.0% P2O5, 74.5% SiO2, 1.2 ppm Cd and 1.6% X2O. The composited feed indicates that there is about 5% of naturally occurring slimes (− 38 μm) at approximately 15% P2O5 deportment and hence, a sacrifice in recovery is expected. This fraction contains a higher contained deleterious element content of approximately 12% X2O3 and thus renders itself to scalping.
Phosphate is present as carbonate fluorapatite. The apatite in the sample is typically well liberated with limited association in the form of Si, Fe, Al and S micro-inclusions.
Reverse cationic flotation was unable to achieve target upgrade specification. It was deduced that the wide size distribution coupled with the slimes adversely affected float performance.
The combined effect of the direct-reverse Crago flowsheet shows that P2O5 can be upgraded to a final concentrate of 32.1% P2O5 at an overall recovery of 61.0% higher than the recovery achieved for the reverse cationic float test reporting at 59.0%. The contaminant specification was achieved at 2.8% X2O3. Perhaps further optimisation tests considering alternative reagents need to be considered to improve on overall recovery as this material is a viable source required to maximise resource utilisation and extend life of mine (LOM).
References
Kropz PLC (2020) Kropz Elandsfontein - A new source of quality phosphate roack. Available at: https://file:///C:/Users/AshmaS/Downloads/kropz-marketing-fact-sheet(3).pdf. Accessed 4 May 2021
Kropz Annual Report (2020) Kropz plc annual report and accounts for the year ended 31 December 2020. Available from: https://www.annualreports.com/HostedData/AnnualReportArchive/k/LSE_KRPZ_2020.pdf. Accessed 22 Feb 2021
Guan C (2009) Theoretical background of the crago phosphate flotation process. Miner Metall Process 26(2):55–64. https://doi.org/10.1007/bf03403419
Zhang JP, Yu Y, Bogan M (1997) Challenging the “Crago” double float process II. Amine -fatty acid flotation of siliceous phosphates. Min Eng 10:983–994
Acknowledgements
The authors would like to acknowledge Mintek for funding the research project and postgraduate study and for providing the necessary facilities and resources to successfully complete the study. Data from this manuscript will be presented at the MINEXCHANGE 2023 SME Annual Conference and Expo, February 26–March 1, 2023 to be held in Denver, Colorado. In addition, the author would like to acknowledge Kropz Elandsfontein for providing material and guidance during testwork.
Funding
Open access funding provided by Mintek.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ashma Singh is currently pursuing a PhD in Chemical Engineering through the University of KwaZulu Natal in South Africa. The work cited within this manuscript is a result of testing conducted at Mintek (where the main author is employed). Research and bursary grant to pursue this work is received from Mintek. The author declares that there is no conflict of interest regarding the work or findings. Dr Jon Pocock is the main author’s supervisor towards her PhD and hence has no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, A., Pocock, J. The ‘Crago Process’ and Its Applicability to a South African Sedimentary Phosphate Deposit. Mining, Metallurgy & Exploration 40, 893–900 (2023). https://doi.org/10.1007/s42461-023-00760-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-023-00760-y