Abstract
The fast depletion of critical metals in natural reserves against their increasing demands in advanced technology application presents the necessity to exploit the end-of-life/waste materials as unconventional resources. Due to a higher accumulation of platinum-group metals (PGMs) in exhausted autocatalytic converters, their recycling through an integrative bio-solvo-chemical technique has been studied. PGMs were efficiently dissolved in bio-cyanide solution produced by Chromobacterium violaceum. The autoclave leaching was optimized in the conditions of temperature, 150°C; pO2, 200 psi; and time, 120 min, yielding > 90% PGMs’ dissolution. PGMs’ separation from cyanide leach liquor was performed using an ionic liquid, Cyphos IL101. Under optimum conditions (i.e., ionic liquid concentration, 0.15 mol/L; extraction pH, 10.4; and temperature, 25°C), Pt and Pd were selectively stripping with > 99% efficiency in 0.1 mol/L (acidic) thiourea and 1.0 mol/L HNO3 solution, respectively, leaving Rh in the raffinate.
Similar content being viewed by others
Introduction
Autocatalytic converters are refractory in nature due to the large mass fraction (on average about 43 wt.%) of cordierite ceramic (Mg2Al4Si5O18) therein. They are usually applied to provide the stable basic skeleton in the honeycomb shape of catalytic converters.1 A thin porous layer of γ-Al2O3 wash-coated onto cordierite provides an effective surface area for dispersing the active metals for the catalytic conversion of exhausted emissions to their less harmful substance (i.e., CO2, N2, and H2O).2,3 Due to the exhibition of the desired oxidation performance along with better thermal stability, the platinum-group metals (PGMs, that include Pt, Pd, and Rh) have been applied since the inception of automotive catalysts for capturing the gaseous emissions.4,5 On average, a total of about 2.0 g/kg of catalysts with individual concentration ranges of 0.3–1.0 g/kg Pt, 0.2–0.8 g/kg Pd, and 0.05–0.1 g/kg Rh (depending on the type of the vehicle) is engineered in a new car.6 The indispensable role of PGMs in autocatalytic converters are leading to the consumption about 35% Pt and over 80% Pd and Rh of their total market demands in 2019.7 In recent times, although the COVID-19 outbreak may affect near-term automobile demand, the long-term demands of PGMs will continue to grow unless substituted by other metals.8 The soaring demands for PGMs and other industrial applications are therefore challenging to alleviate their supply risks with the limited natural reserves and depletion of high-grade ores.9 Henceforth, PGMs’ extraction from end-of-life (EoL) materials as unconventional resources like exhausted autocatalytic converters is of paramount interest because their concentration in exhausted catalysts (about a total of 0.1 wt.%) is a thousand times higher than that of the primary ores.10,11 In parallel, the treatment of EoL materials can significantly reduce the waste volume and lower the economic growth dependency on primary minerals’ processing.11
Due to the refractory nature of catalytic converters, high-temperature smelting for collecting PGMs with iron or copper has been employed.1 This process is highly energy-consuming and discharges gaseous emissions, which is followed by the tedious dissolution and separation works resulting in unavoidable losses of PGMs.12 Due to this, hydrometallurgical recycling has attracted researchers.13 Usually, mineral acids of high concentrations including aqua regia and several additives (i.e., H2O2 and Al/Cu chloride) have been employed for PGM dissolution,1,11,14,15,16 albeit the high reagent consumption, the generation of highly acidic effluents, the instability of PGM extraction, and the low recovery of Rh have been found to be unsatisfactory.17 Recently, biotechnology has emerged as a good alternative with a low carbon footprint in extractive metallurgy from ores;18,19 however, it is still in the infancy stage for PGM recycling from refractory materials like autocatalytic converters as they are hard-to-leach noble metals.
PGMs’ property to form cyanide complexes has been utilized by some researchers with low efficiency of Pt and Pd at about 80–85%, and Rh at around 70–75%.20,21 To the best of our knowledge, no effective bio-cyanidation-leaching of PGMs has yet been reported except for some simulated chemical leaching with NaCN instead of using actual biogenic cyanide in the system. Therefore, the investigation of PGMs’ bio-cyanidation-leaching from the exhausted autocatalytic converter is imperative. Furthermore, selective recovery of PGMs from cyanide leach liquor by adsorption with activated carbon22,23,24 and reductive precipitation with zinc25 has been frequently studied. Activated carbon exhibited poor operating ability due to prolonged adsorption with small sorption capacity and high elution temperature.26 Due to the low concentration of PGMs, cementation by Zn-dust seems to be impractical.25 Nevertheless, liquid-liquid extraction has been recently found to be attractive in precious metal metallurgy due to fast extraction kinetics with high capacity and potential regeneration of organic solvents. Very few extractants have been identified to be suitable for PGM extraction from an alkaline cyanide solution, including “green solvents” ionic liquids.25 In recent times, several ionic liquids (e.g., pyridinium, pyrrolidinium, and piperidinium, betainium, and phosphonium) have been applied,27 although none of the studies has dealt with biogenic cyanide medium.
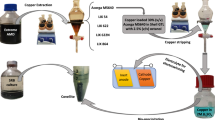
In the present study, bio-cyanidation of the exhausted autocatalytic converter was examined using oxidative decarboxylation of glycine for the HCN synthesis during the growth phase of Chromobacterium (C.) violaceum.28,29 The ground sample preconditioned with NaOH solution was leached with different concentrations of biogenic cyanide under elevated temperature and partial oxygen pressure (pO2). Further, PGMs’ separation from the biocyanide leach liquor was performed with an ionic liquid trihexyl(tetradecyl)phosphonium chloride (Cyphos IL101). It is noteworthy to mention that, due to an excellent and simple regeneration ability of Cyphos IL101,27 it was chosen to be used in this study. Further, the effect of ionic liquid concentration, extraction pH, temperature, and behavior of the stripping reagents was examined to optimize the conditions of the liquid–liquid extraction process.
Materials and Methods
Materials
An amount of 15 kg of exhausted autocatalytic converters was procured from a supplier in Korea. The procured catalysts were ground to size ~ 150 µm using a locally made pulveriser machine. The ground sample was homogenized before preconditioning in 2.0 mol/L NaOH solution at 90°C for 60 min. The preconditioned mass was sent for analysis after a hot water washing and overnight drying in a hot air oven at 80°C. X-ray fluorescence spectroscopy of the preconditioned mass, which was fed for pressure leaching, yielded the composition of refractory substances to be Al2O3 41.8%, MgO 17.4%, SiO2 24.2%, CeO2 5.34%, ZrO2 0.72%, and TiO2 0.56%. Fire-assay analysis of the preconditioned sample revealed the PGM contents to be Pt 756 ppm, Pd 367.5 ppm, and Rh 170.8 ppm. Reagents like NaOH pellets (Junsei Chemical), hydrochloric acid (Merck), nitric acid (Riedel-deHa¨en), thiourea (Junsei Chemical), the ionic liquid trihexyl(tetradecyl)phosphonium chloride, namely Cyphos IL101 (Cytec Industries, Canada), and aromatic solvent C10 (SOLVENTIS) were used without further purification.
Methods
HCN Synthesis and Bio-Cyanide Production
A cyanide-producing bacterium, C. violaceum DSM30191T, was maintained in YP-medium (yeast extract 5 g, peptone 10 g, distilled water 1 L) at 30°C. For continuous cyanide production and accumulation, the bioreactor was supplemented with 2.0 g/L glycine, 1 L of YP-medium, and two collectors containing 3.5 mol/L NaOH solutions under an agitation of 300 rpm by magnetic paddles. Aeration under the flow rate of 3 mL/s was supplied to the biological system. Then, 12 mL of pre-grown C. violaceum (approximately 106 CFU/mL) was inoculated into a bioreactor at 30°C without feeding fresh YP-medium for 1 day, and pH was adjusted at 8.5. After day 1, a fresh YP medium (containing 2.0 g/L glycine) was fed continuously at a 1000-mL/day flow rate with a peristaltic pump. The reaction volume was constantly maintained at 1 L with a 1-day hydraulic retention time, and the reactor was operated for 16 days. After periodic sample collection from the bioreactor and the cyanide accumulation reactors (bottles), the same volume of fresh NaOH solution was added to maintain the total reaction volume. Counts of viable cells were carried out by the drop plate method of serial dilution on nutrient agar. The plates were incubated for 24 h at 30°C. The cyanide concentration was analyzed by titration against a standard AgNO3 solution. After 16 days of cyanide entrapped in NaOH solution, a total of 5.8 g/L NaCN was obtained in the solution of pH 11.6. This biogenic cyanide solution was used as the lixiviant medium for PGM dissolution by autoclave leaching under high pressure and temperature.
Autoclave Leaching for PGMs’ Dissolution
Pressure leaching studies of the preconditioned sample were conducted in a 500-mL Parr autoclave with a (Ni-Cr) Alloy 600 reactor, a variable speed stirrer equipped with two axial impellers with 6 blades, and a controller outside along with an internally mounted cooling coil. Then, a 40-g sample in 200 mL of the biogenic cyanide solution of a predetermined concentration was charged in the reactor under the fixed agitation speed of 200 rpm and duration of 1 h, while the temperature, pressure, and NaCN concentration were varied. To vary the NaCN concentration, the duration of the biocyanidation process was accordingly adjusted along with maintaining pH 11.6 as invariable. The oxygen pressure was maintained by externally supplying the industrial-grade O2 after reaching the desired temperature and considering the in situ pressure at that temperature. At the end of the experiment, the oxygen supply was stopped, and the autoclave was water-cooled. Then, the solution was filtered using a 0.45-μm membrane filter, collecting the filtrate for PGM analysis using inductively coupled plasma–mass spectrometry (ICP-MS; MSS 01; SPECTRO Analytical Instruments, Germany). The leaching efficiency of the metals was calculated by:
where MIS and MLL are the metal contents in the solid sample and leach liquor, respectively.
PGMs’ Separation from Leach Liquor
Liquid–liquid separation studies were performed in 20-mL vials, wherein equal volumes (6 mL) of leach liquor and 0.15 mol/L ionic liquid solution (otherwise varied in the range of 0.01–0.15 mol/L concentration) were equilibrated at 25 ± 1°C for 10 min. Both phases were settled by centrifugation for 3 min, and then the raffinate was carefully withdrawn to analyse the PGM concentration using ICP-MS. As per the analysis of the raffinate, the distribution ratio (D) of the metal was calculated as:
where CEorg and CEaq are the post-extraction metal concentration in the organic and aqueous phases, respectively. Using the D value, the extraction efficiency (in %) was determined as:
where Vorg and Vaq are the volumes of the organic and aqueous phases, respectively.
Further, the stripping of Pt and Pd from the loaded ionic liquid was performed by contact with the pre-determined concentration of stripping solutions at an O:A of 1:1, temperature 25 ± 1°C, and contact time of 10 min. after the phase separation. The stripping efficiency was calculated as:
where CSorg and CSaq are the post-stripping metal concentrations in the organic and aqueous phases, respectively.
Results and Discussion
Pressure Leaching in Bio-Cyanide Solution
Effect of Temperature
Temperature has a direct relationship with pressure for the enhancement of the rate of reaction.30 Hence, pressure leaching of PGMs using a biogenic cyanide lixiviant was first investigated at different temperatures elevated in the range of 100–200°C, while keeping the other parameters constant (NaCN concentration, 5.8 g/L; pO2, 150 psi; initial pH, 11.6; and time, 60 min). The results shown in Fig. 1 depict a positive effect of temperature on PGM dissolution. As can be seen, at the elevated temperature of 100°C and oxygen pressure of 150 psi, PGM dissolution in the cyanide solution was not more than 85%. An increasing trend of metal dissolution can be ascribed to the physical phenomenon of increased collisions between the particles of the heterogeneous system.30,31 Dissolution efficiency reached the maximum of 91% Pt, 94% Pd, and 87% Rh at 150°C; thereafter, increasing temperature showed a decline in PGM dissolution (87% Pt, 81% Pd, and 84% Rh at 150°C). Comparatively, Pt and Rh dissolution decreased slightly in contrast to a steep decline of Pd, which can be corroborated by the non-stability of Pd(CN)42− and by cyanide decomposition at higher temperatures.17
Effect of Partial Oxygen Pressure
For autoclaving, in particular, to dissolve difficult-to-leach metals from a refractory material, the role of pressure becomes crucial to liberate the desired metals into solution. Hence, PGM dissolution in biogenic cyanide was examined at various oxygen pressures in the range of 50–250 psi at a fixed temperature of 150°C. The results in Fig. 2 depict an increasing trend for PGM dissolution with elevating oxygen pressure in the closed system. Bio-cyanide leaching of PGMs increases significantly (Pt, 83% to 94%; Pd, 86% to >96%; and Rh, 76% to ~90%) with a change in pO2 from 50 psi to 250 psi. This behavior can be understood by breaking the surface chemical barrier to attack directly the PGM particles by the biogenic cyanide solution, which enhances the dissolution efficiency.11 Moreover, the catalytic role of oxygen in cyanide can also be corroborated by the exhibited behavior through the cyanidation reactions, as given in Eqs. 5–7.17 Since the maximum dissolution was achieved with a pO2 of 200 psi, it was maintained throughout the experimental sets.
Effect of NaCN Concentration
To form the PGM cyanide, the optimization of the cyanide concentration for driving Eqs. 5–7 was carried out in the range of 0.63–6.43 g/L NaCN at a constant temperature (150°C) and pO2 (200 psi). As shown in Fig. 3, PGM dissolution progressed with increasing cyanide concentration as the lixiviant medium. Bio-cyanide leaching of PGMs changed significantly (Pt, from 73% to > 95%; Pd, from 82% to 96%; and Rh, from 68% to ~ 91%) with NaCN concentration from 0.63 g/L to 6.43 g/L. This change in the PGM dissolution behavior indicates a change in the rate mechanism from diffusion-controlled at low cyanide concentration to the chemically-controlled at higher cyanide in solution.11,30,31 The maximum leaching efficiency (Pt, 157.5 mg/L; Pd, 77.6 mg/L; and Rh, 33.9 mg/L) was achieved at 5.82 g/L NaCN solution captured through the microbial-driven HCN synthesis. Thus, this concentration was consider optimum to generate the stock leach liquor for subsequent recovery studies using an ionic liquid (Cyphos IL101).
PGM Separation from Leach Liquor Using Ionic Liquid
Effect of Ionic Liquid Concentration
To study the extraction behavior of PGM’ cyanide complexes with the phosphonium-based ionic liquid, Cyphos IL101, further experiments were conducted in the concentration range of 0.01–0.15 mol/L Cyphos IL101 prepared in aromatic solvent C10. Other parameters like the O:A phase ratio (1:1), contact time (10 min), and temperature (25°C) were kept constant while contacting the leach liquor of pH 10.8. Figure 4 shows that Pt and Pd extraction increased with more active molecules of ionic liquid in the organic phase, leaving all the Rh in the aqueous raffinate. The shifting of distribution curves with the availability of more extraction sites during the contact of two different phases can be corroborated by the increasing extraction of Pt and Pd.32,33 However, the selectivity for Rh remaining non-extractable in the raffinate can be related to the geometrically octahedral structure of Rh(CN)63− in comparison to the square planar structure of Pt(CN)42− and Pd(CN)42− complexes.17 The extraction of Pt and Pd with Cyphos IL101 can be written as Eqs. 8 and 9. As can be seen, the extraction efficiency of Pt and Pd improved from approximately 23% to 97% and < 18% to 94% with increasing concentration of ionic-liquid from 0.01 mol/L to 0.15 mol/L, respectively. Hence, a concentration 0.15 mol/L Cyphos IL101 was optimized to use in the next sets of experiments.
Effect of Leach Liquor pH
To evaluate the effect of pH on PGM extraction, the pH value of the leach liquor was varied from 9.6 to 11.2 while contacting with 0.15 mol/L ionic liquid solution at an O:A ratio of 1. Figure 5 depicts an increasing trend with Pt and Pd extraction by decreasing the pH of the leach liquor from 11.2 (96.4% Pt and 92.7% Pd) to 10.4 (98.2% Pt and 97.6% Pd). Thereafter, the extraction efficiency was unchanged up to the leach liquor’s pH of 9.6. A lower extraction at the higher alkaline pH of leach liquor was probably caused by the competitive extraction of OH– ions.34 To prevent the toxicity of HCN at lower pH,28 the extraction at pH 10.4 was found to be quantitative and was optimized for subsequent experiments.
Effect of Temperature
PGM extraction with ionic liquid Cyphos IL101 was evaluated at different temperatures between 25°C and 55°C while contacting the two phases at an O:A phase ratio of 1:1. Figure 6a shows declining extractions from 98% to < 90% Pt and ~ 97% to 87% Pd with respect to increasing temperature from 25°C to 55°C. This behavior, indicative of the exothermic process, reveals that the ability of metal-binding sites of Cyphos IL101 is destroyed with the increasing temperature.35,36 In the above sets of experiments, Rh was not extracted in the organic phase.
Selective Stripping of Pt and Pd from Loaded Ionic Liquid
The Pt- and Pd-loaded ionic liquid was subjected to selective stripping to achieve their value-added products' high purity from the recycling process. In this context, two different reagents were chosen to investigate the stripping behaviour of the metals. The solutions of 0.1 mol/L CH4N2S (prepared in 0.5 mol/L HCl) and 1.0 mol/L HNO3 were separately contacted with the loaded organic phase at an O:A of 1, contact time of 10 min, and 25°C. The results are shown in Fig. 7, which reveals the possible selective stripping of Pt and Pd. Stripping with thiourea (refer to Fig. 7a) shows that ~ 92% Pd recovery in aqueous solution can be achieved in one step of contact, which accumulatively reached > 99% after two steps of stripping along with 1.4% of Pt. In the case of stripping with nitric acid (refer to Fig. 7b), Pt was selectively stripped back in acidic solution, albeit with only 73% efficacy. Therefore, the quantitative stripping of Pt was investigated in 3 steps for an accumulative efficiency of ~ 99%. In this manner, the separation between Pt and Pd could be achieved by stripping of the co-extracted loaded ionic liquid phase.
Conclusion
An efficient recycling of a postconsumer refractory material (i.e., exhausted autocatalytic converter) has been studied for PGM recovery by applying an integrative bio-solvo-chemical approach. In order to liberate the PGM particles from the refractory matrix, a NaOH-preconditioned sample was used for the pressure leaching with a biogenic cyanide solution of NaCN produced through HCN synthesis during the growth phase of C. violaceum. The pressure autoclave leaching of PGMs with the biogenic cyanide solution yielded the maxima of 91% Pt, 94% Pd, and 87% Rh at 150°C, which remarkably increased with pO2 250 psi (94% Pt, > 96% Pd, and ~ 90% Rh) and 5.82 g/L NaCN concentration in the biogenic solution. Thereafter, PGM separation from the pressure-leached liquor was demonstrated with an ionic liquid, Cyphos IL101. Selective extraction of Pt and Pd over Rh was achieved under the optimum conditions of 0.15 mol/L Cyphos IL101 with initial leach liquor pH 10.4 and temperature of 25°C. Further, the separation of Pt and Pd was achieved by the selective stripping of loaded ionic liquid using thiourea and nitric acid solutions. A solution of 0.1 mol/L CH4N2S (prepared in 0.5 mol/L HCl) was contacted in two steps followed by contacting the Pd-depleted organic phase with 1.0 mol/L HNO3 in three steps which could recover > 99% Pt and Pd, respectively. The process essentially contributes to the establishing of an efficient recycling of PGMs coupled with their circular economy to provide an unconventional route of economic development than that by using the primary resources alone.
References
S. Ilyas, R.R. Srivastava, H. Kim, and H.A. Cheema, Sep. Purif. Technol. 248, 117029 (2020).
M.P. Singh, and S. Raj, IJSER 7, 927 (2016).
E.S. Lox, Automotive exhaust treatment, in Handbook of Heterogeneous Catalysis. ed. by G. Ertl, H. Knözinger, F. Schüth, and J. Weitkamp (Wiley, Weinheim, 2008), p. 2274.
A. Russell, and W.S. Epling, Catal. Rev. 53, 337 (2011).
M.V. Twigg, Catal. Today 117, 407 (2006).
H.B. Trinh, J. Lee, Y. Suh, and J. Lee, Waste Manag. 114, 148 (2020).
Johnson Matthey PGM Market Report. (2020). http://www.platinum.matthey.com/services/market-research/pgm-market-reports Accessed from 26 Apr 2020.
N. Jonson and F. Warwick, Palladium, rhodium demand to remain, despite virus outbreak: analyst. (2021). https://www.spglobal.com/platts/en/market-insights/latest-news/metals/030520-palladium-rhodium-demand-to-remain-despite-virus-outbreak-analyst Accessed from 28 Apr 2021.
KPMG Commodity Insights Bulletin Series, Commodity Insights Bulletin - KPMG Global (home.kpmg). (2015). Accessed from 26 May 2021.
S. Steinlechner, and J. Antrekowitsch, PGM recycling from catalysts in a closed hydrometallurgical loop with an optional cerium recovery, in Enabling Materials Resource Sustainability. ed. by A. Kvithyld, C. Meskers, R. Kirchain, G. Krumdick, B. Mishra, M. Reuter, C. Wang, M. Schlesinger, G. Gaustad, D. Lados, and J. Spangenberger (Springer, Cham, 2013), p. 361.
S. Ilyas, H. Kim, and R.R. Srivastava, Sustainable Urban Mining of Precious Metals (CRC, Boca Raton, 2021).
J. Chen, K. Huang, and Y.R. Chen, South African Patent 2005/05141 (2005).
G. Nicol, E. Goosey, D.Ş Yıldız, E. Loving, V.T. Nguyen, S. Riaño, I. Yakoumis, A.M. Martinez, A. Siriwardana, A. Unzurrunzaga, J. Spooren, T.A. Atia, B. Michielsen, X. Dominguez-Benetton, and O. Lanaridi, Johns. Matthey Technol. Rev. 65, 127 (2021).
A. Fornalczyk, M. Kraszewski, J. Willner, J. Kaduková, A. Mrážiková, R. Marcinčáková, and O. Velgosová, Arch. Metall. Mater. 61, 233 (2016).
C.A. Nogueira, A.P. Paiva, P.C. Oliveira, M.C. Costa, and A.M. Rosa da Costa, J. Hazard. Mater. 278, 82 (2014).
S. Steinlechner, and J. Antrekowitsch, JOM 67, 406 (2015).
J. Chen, and K. Huang, Hydrometallurgy 82, 164 (2006).
S. Ilyas, R. Chi, H.N. Bhatti, I.A. Bhatti, and M.A. Ghauri, Bioprocess. Biosyst. Eng. 35, 433 (2012).
J. You, S.K. Solongo, A. Gomez-Flores, S. Choi, H. Zhao, M. Urik, S. Ilyas, and H. Kim, Bioresour. Technol. 307, 123181 (2020).
G.B. Atkinson, US Patent 5160711 (1992).
D.P. Desmond, High-temperature cyanide leaching of platinum group metals from automobile catalysts — laboratory test. In: Report of Investigations, vol. RI-9384, United States Bureau of Mines (1991).
C.A. Snyders, S.M. Bradshaw, G. Akdogan, and J.J. Eksteen, Hydrometallurgy 149, 132 (2014).
C.A. Snyders, S.M. Bradshaw, G. Akdogan, and J.J. Eksteen, Miner. Eng. 80, 14 (2015).
C.N. Mpinga, S.M. Bradshaw, G. Akdogan, C.A. Snyders, and J.J. Eksteen, Miner. Eng. 55, 11 (2014).
C.N. Mpinga, S.M. Bradshaw, G. Akdogan, C.A. Snyders, and J.J. Eksteen, Hydrometallurgy 142, 36 (2014).
C. Jin, M. Chen, M. Fan, G. Luo, M. Shao, Z. Huang, and X. Xie, J. Mol. Liq. 336, 116358 (2021).
S. Ilyas, H. Kim, and R.R. Srivastava, Sep. Purif. Technol. 248, 119577 (2022).
S. Ilyas, and N. Ilyas, Microbial cyanidation of gold, in Gold Metallurgy and the Environment. ed. by S. Ilyas, and J. Lee (CRC, Boca Raton, 2018), p. 157.
R.R. Srivastava, S. Ilyas, H. Kim, S. Choi, H.B. Trinh, M.A. Ghauri, and N. Ilyas, J. Chem. Technol. Biotechnol. 95, 2796 (2020).
F. Habashi, Principles of Extractive Metallurgy, vol I. (Gordon and Breach, New York, 1969).
H. Munir, R.R. Srivastava, H. Kim, S. Ilyas, M.K. Khosa, and B. Yameen, J. Chem. Technol. Biotechnol. 95, 2286 (2020).
G.M. Ritcey, and A.W. Ashbrook, Solvent Extraction Part I (Elsevier, Amsterdam, 1984).
R.R. Srivastava, S. Ilyas, H. Kim, N.L.M. Tri, N. Hassan, M. Mudassir, and N. Talib, JOM 72, 839 (2020).
Z.Y. Wang, Y. Sun, N. Tang, C.L. Miao, Y.T. Wang, L.H. Tang, S.X. Wang, and X.J. Yang, Sep. Purif. Technol. 222, 136 (2019).
V.S. Kislik, Solvent Extraction: Classical and Novel Approaches (Elsevier, Amsterdam, 2012).
S. Ilyas, H. Kim, and R.R. Srivastava, JOM 73, 19 (2021).
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Project No. 2020R1I1A1A01074249).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ilyas, S., Kim, H. Recovery of Platinum-Group Metals from an Unconventional Source of Catalytic Converter Using Pressure Cyanide Leaching and Ionic Liquid Extraction. JOM 74, 1020–1026 (2022). https://doi.org/10.1007/s11837-021-05119-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05119-6