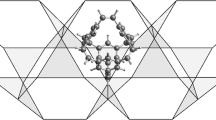
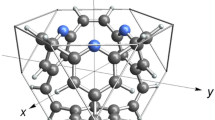
Abstract
Ionic hydrocarbon compounds that contain hypercarbon atoms, which bond to five or more atoms, are important intermediates in chemical synthesis and may also find applications in hydrogen storage. Extensive investigations have identified hydrocarbon compounds that contain a five- or six-coordinated hypercarbon atom, such as the pentagonal-pyramidal hexamethylbenzene, C6(CH3)62+, in which a hexacoordinate carbon atom is involved. It remains challenging to search for further higher-coordinated carbon in ionic hydrocarbon compounds, such as seven- and eight-coordinated carbon. Here, we report ab initio density functional calculations that show a stable 3D hexagonal-pyramidal configuration of tropylium trication, (C7H7)3+, in which a heptacoordinate carbon atom is involved. We show that this tropylium trication is stable against deprotonation, dissociation, and structural deformation. In contrast, the pyramidal configurations of ionic C8H8 compounds, which would contain an octacoordinate carbon atom, are unstable. These results provide insights for developing new molecular structures containing hypercarbon atoms, which may have potential applications in chemical synthesis and in hydrogen storage.

Possible structural transformations of stable configurations of (C7H7)3+, which may result in the formation of the pyramidal structure that involves a heptacoordinate hypercarbon atom.






Similar content being viewed by others
References
Olah GA (1995) Angew Chem Int Ed 34:1393–1405
Olah GA, Prakash G, Wade K, Molnár Á, Williams RE (2011) Hypercarbon chemistry, 2nd edn. Wiley, Hoboken, p 85–147
Du J, Sun X, Jiang G, Zhang C (2016) Int J Hydrog Energy 41:11301–11307
Gao Y, Shao N, Zhou RL, Zhang GL, Zeng XC (2012) J Phys Chem Lett 3:2264–2268
Hogeveen H, Kwant PW (1975) Acc Chem Res 8:413–420
Surya Prakash G, Rasul G, Olah GA, Phys J (1998) J Phys Chem A 102:2579–2583
Olah GA, Rasul G (1997) Acc Chem Res 30:245–250
Boo DW, Lee YT (1995) J Chem Phys 103:520–530
Williams RE (1971) Inorg Chem 10:210–214
Stohrer WD, Hoffmann R (1972) J Am Chem Soc 94:1661–1668
Masamune S, Sakai M, Ona H, Jones AJ (1972) J Am Chem Soc 94:8956–8958
Kemp-Jones AV, Nakamura N, Masamune S (1974) J Chem Soc Chem Commun 109–110
Masamune S, Sakai M, Kemp-Jones A, Ona H, Venot A, Nakashima T (1973) Angew Chem Int Ed 12:769–771
Coates R, Fretz E (1977) Tetrahedron Lett 18:1955–1960
Hogeveen H, Kwant PW (1974) J Am Chem Soc 96:2208–2214
Hogeveen H, Kwant PW (1973) Tetrahedron Lett 14:1665–1670
Jašík J, Gerlich D, Roithová J (2014) J Am Chem Soc 136:2960–2962
Lammertsma K, Barzaghi M, Olah GA, Pople JA, Schleyer P, Simonetta M (1983) J Am Chem Soc 105:5258–5263
Lammertsma K, Olah GA, Barzaghi M, Simonetta M (1982) J Am Chem Soc 104:6851–6852
Jursic BS (1999) J Chem Res Synop :502–503
Malischewski M, Seppelt K (2017) Angew Chem Int Ed 56:368–370
Malischewski M, Seppelt K (2017) Angew Chem Int Ed 56:16495–16497
Rasul G, Prakash GS, Olah GA (2010) J Phys Chem A 114:12124–12127
Olah GA, Rasul G (1996) J Am Chem Soc 118:8503–8504
Kresse G, Furthmuller J (1996) Phys Rev B 54:11169–11186
Payne MC, Teter MP, Allan DC, Arias T, Joannopoulos J (1992) Rev Mod Phys 64:1045
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Blochl PE (1994) Phys Rev B 50:17953–17979
Kresse G, Joubert D (1999) Phys Rev B 59:1758–1775
Henkelman G, Uberuaga BP, Jonsson H (2000) J Chem Phys 113:9901–9904
Bader RFW (1990) Atoms in molecules - a quantum theory. Clarendon, Oxford
Henkelman G, Arnaldsson A, Jónsson H (2006) Comput Mater Sci 36:354–360
Von W, Doering E, Knox L (1957) J Am Chem Soc 79:352–356
Klein JEMN, Havenith RWA, Knizia G (2018) Chem Eur J. https://doi.org/10.1002/chem.201705812
Hückel E (1931) Z Phys 70:204–286
Roberts JD, Streitwieser Jr A, Regan CM (1952) J Am Chem Soc 74:4579–4582
Jemmis ED, Schleyer P v R (1982) J Am Chem Soc 104:4781–4788
McKee WC, Agarwal J, Schaefer HF, Schleyer P v R (2014) Angew Chem Int Ed 53:7875–7878
Acknowledgments
The authors would like to thank Michael Lee from the Oklahoma School of Science and Mathematics (now at the University of Oklahoma) for valuable discussions at the early stage of this work. The calculations have been performed using computational resources at the OU Supercomputing Center for Education & Research (OSCER) at the University of Oklahoma.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1941 kb)
Rights and permissions
About this article
Cite this article
Wang, G., Rahman, A.K.F. & Wang, B. Ab initio calculations of ionic hydrocarbon compounds with heptacoordinate carbon. J Mol Model 24, 116 (2018). https://doi.org/10.1007/s00894-018-3640-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3640-9