Abstract:
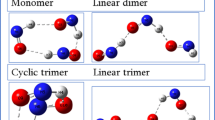
Characteristic properties as well as possible differences in bonding of small group 12 clusters (, Cd, Hg; ) have been investigated by quantum chemical ab initio methods, i.e., relativistic large-core pseudopotentials, core-polarization potentials and coupled-cluster correlation treatments. A comparison of cohesive energies and spectroscopic properties like ionization potentials, electron affinities, and vibrational frequencies reveals a close similarity between the clusters of Cd and Hg. For Zn clusters we observed an exceptional increase in stability between and . In order to get a more qualitative picture of the covalent contributions to bonding we have calculated the electron localization function (ELF). The ELF analysis is in accordance with the calculated spectroscopic properties and shows predominant van der Waals interactions with weak covalent contributions for all the cluster sizes considered.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 23 July 1998 / Received in final form: 11 January 1999
Rights and permissions
About this article
Cite this article
Flad, HJ., Schautz, F., Wang, Y. et al. On the bonding of small group 12 clusters. Eur. Phys. J. D 6, 243–254 (1999). https://doi.org/10.1007/PL00021622
Issue Date:
DOI: https://doi.org/10.1007/PL00021622