Summary.
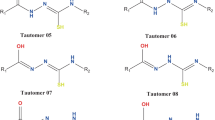
Using 2D ROESY 1H NMR spectroscopy it could be unequivocally shown from nuclear Overhauser effects and intramolecular exchange correlations of strategic signals that hypericin as well as its 3-hypericinate ion are present as the tautomers with the carbonyl groups located in positions 7 and 14 in polar solvents like dimethylsulfoxide. In apolar solvents like tetrahydrofuran hypericin prevails as the 1,6-dioxo tautomer.
Zusammenfassung.
Mit Hilfe der 2D-ROESY-1H-NMR-Spektroskopie konnte über Kern-Overhauser-Effekte und Austauschkorrelationen strategischer Signale zweifelsfrei nachgewiesen werden, daß Hypericin und sein 3-Hypericination in polaren Lösungsmitteln wie Dimethylsulfoxid als Tautomere mit den Carbonylgruppen in Positionen 7 und 14 vorliegen. In unpolaren Lösungsmitteln wie Tetrahydrofuran dominiert das 1,6-Tautomere.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received February 4, 1999. Accepted March 1, 1999
Rights and permissions
About this article
Cite this article
Dax, T., Falk, H. & Kapinus, E. A Structural Proof for the Hypericin 1,6-Dioxo Tautomer. Monatshefte fuer Chemie 130, 827–831 (1999). https://doi.org/10.1007/PL00010264
Published:
Issue Date:
DOI: https://doi.org/10.1007/PL00010264