Abstract
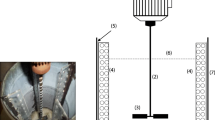
In this study, the effects of stirring speed (80, 180, 400 and 600 rad/min), propeller type (two-, four- and six-bladed), solution temperature (25° and 60°C) and initial silver concentration (10 and 40 g/L) on the recovery of silver from silver nitrate solutions by copper were investigated. A cementation system with forced convection was developed to overcome the problems observed in technological applications where copper particles adhere together and cement silver encapsulates copper. The effects of various conditions of the forced convection system on cement silver morphology, product quality and cementation efficiency were examined. Experimental results show that more than 98% cementation efficiency can be reached within two hours by applying effective stirring (> 400 rad/min). Silver ion concentration decreases as the stirring speed increases (4,312 ppm at 80 rad/min compared to 1.85 ppm at 600 rad/min after 4 hrs). The cementation rate was determined by the grain size and adhesion strength of the first and second layers of the cement silver. It was ascertained that the cementation process could be accelerated by creating a mechanical effect so that the cement layer atop copper could break. Thus, in a system of forced convection, the cementation rate becomes independent of the initial concentration and increases with increasing temperature.
Similar content being viewed by others
References
Alemany, C., Diard, J.P., Gorrec, B.L., and Montella, C., 1996, “Electrochemical impedance spectroscopy study of silver cementation on copper,” Electrochimica Acta, Vol. 41, No. 9, pp. 1483–1489.
Glicksman, R., Mouquin, H., and King, C.V., 1953, “Rate of displacement of silver from aqueous silver nitrate by zinc and copper,” Journal of The Electrochemical Society, Vol. 100, No. 12, pp. 580–585.
Gurmen, S., Timur, S., and Duman, I., 1997, “Electrolytical separation of precious metal alloys,” Proc. of the 9th International Metallurgy and Materials Congress, Istanbul, 11–15 June, pp. 1009–1013 (in Turkish).
Manziek, L., 1990, Precious Metals Recovery and Refining, Historical Publications, Texas, pp. 83–93.
Morrison, R.M., Mackinnon, D.J., and Brannen, J.M., 1987, “Silver cementation from chloride solution using rotating disk of copper and lead,” Hydrometallurgy, Vol. 18, pp. 207–223.
Ritchie, I.M., and Robertson, S.G., 1997, “A capacitance study of the silver(l)/copper displacement reaction,” Journal of Applied Electrochemistry, Vol. 27, pp. 59–63.
Sedzimir, J.A., 2002, “Precipitation of metals by metals (cementation)-kinetics, equilibria,” Hydrometallurgy, Vol. 64, pp. 161–167.
Sulka, G.D., and Jaskula, M., 2002, “Study of the kinetics of the cementation of silver ions onto copper in a rotating cylinder system from acidic sulphate solutions,” Hydrometallurgy, Vol. 64, pp. 13–33.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Timur, S., Cetinkaya, O., Erturk, S. et al. Investigating silver cementation from nitrate solutions by copper in forced convection systems. Mining, Metallurgy & Exploration 22, 205–210 (2005). https://doi.org/10.1007/BF03403324
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03403324