Abstract
Background
The loss of β cells in type 1 diabetes may involve protein kinases because they control cell growth, differentiation, and survival. Previous studies have revealed that GTK, a Src-like protein tyrosine kinase expressed in β cells (also named Bsk/Iyk), regulates multiple responses including growth and survival of rat insulinoma cells (RINm5F) and differentiation of neuronal PC12 cells. In the present study, we have generated a transgenic mouse expressing a kinase active GTK mutant (GTK-Y504F) under the control of the rat insulin I promoter to establish a role of GTK in β cells.
Materials and Methods
Control and GTK-transgenic CBA mice were used for determination of in vivo glucose tolerance and the relative insulin-positive area. Isolated islets from both groups were cultured in the absence and presence of cytokines and insulin secretion, viability and protein expression were assessed.
Results
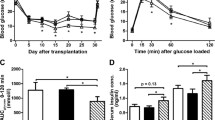
The β-cell mass of GTK-transgenic mice was increased as a consequence of a larger pancreas and an increased relative β-cell area. Islets isolated from the transgenic animals exhibited an enhanced glucose-induced insulin release and reduced viability in response to cytokines that could not be explained by higher levels of nitric oxide (NO) compared with control islets. Extracellular signal-regulated kinase (ERK) 1/2, p38 mitogen-activated protein kinase (MAPK), c-Jun NH2-terminal kinase (JNK), and Akt were all activated by cytokines, but GTK-transgenic islets contained higher basal levels of phosphorylated ERK1/2 and lower basal levels of phosphorylated p38 compared with the control islets. The total amount of activated MAPKs was, however, higher in the cytokine-stimulated transgenic islets compared with the control islets due to increased levels of phospho-ERK1/2. Moreover, the proline-rich tyrosine kinase (PYK) 2 (also named RAFTK/CAK β/CADTK) levels were elevated in response to a 24-hr exposure to cytokines in control islets but not in the GTK-transgenic islets.
Conclusions
These results suggest that although GTK increases the β-cell mass, it also enhances islet cell death in response to cytokines and may thus be involved in the β-cell damage in type 1 diabetes.







Similar content being viewed by others
References
Mauricio D, Mandrup-Poulsen T. (1998) Apoptosis and the pathogenesis of IDDM: a question of life and death. Diabetes 47: 1537–1543.
Rabinovitch A, Suarez-Pinzon WL, Shi Y, Morgan AR, Bleackley RC. (1994) DNA fragmentation is an early event in cytokine-induced islet beta-cell destruction. Diabetologia 37: 733–738.
Rabinovitch A, Suarez-Pinzon WL. (1998) Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem. Pharmacol. 55: 1139–1149.
Rabinovitch A. (1998) An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 14: 129–151.
Finegood DT, Scaglia L, Bonner-Weir S. (1995) Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44: 249–256.
Mandrup-Poulsen T. (1996) The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 39: 1005–1029.
Eizirik DL, Flodström M, Karlsen AE, Welsh N. (1996) The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia 39: 875–890.
Liu D, Pavlovic D, Chen MC, Flodström M, Sandler S, Eizirik DL. (2000) Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS-/-). Diabetes 49: 1116–1122.
Yamada K, Takane-Gyotoku N, Yuan X, Ichikawa F, Inada C, Nonaka K. (1996) Mouse islet cell lysis mediated by interleukin-1-induced Fas. Diabetologia 39: 1306–1312.
Zumsteg U, Frigerio S, Holländer GA. (2000) Nitric oxide production and Fas surface expression mediate two independent pathways of cytokine-induced murine beta-cell damage. Diabetes 49: 39–47.
Suarez-Pinzon W, Sorensen O, Bleackley RC, Elliott JF, Rajotte RV, Rabinovitch A. (1999) Beta-cell destruction in NOD mice correlates with Fas (CD95) expression on beta-cells and proinflammatory cytokine expression in islets. Diabetes 48: 21–28.
Larsen CM, Wadt KA, Juhl LF, et al. (1998) Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J. Biol. Chem. 273: 15294–15300.
Ammendrup A, Maillard A, Nielsen K, et al. (2000) The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes 49: 1468–1476.
Pavlovic D, Andersen NA, Mandrup-Poulsen T, Eizirik DL. (2000) Activation of extracellular signal-regulated kinase (ERK)1/2 contributes to cytokine-induced apoptosis in purified rat pancreatic b-cells [In Process Citation]. Eur. Cytokine Netw. 11: 267–274.
Pousset F, Dantzer R, Kelley KW, Parnet P. (2000) Interleukin-1 signaling in mouse astrocytes involves Akt: a study with interleukin-4 and IL-10. Eur. Cytokine Netw. 11: 427–434.
Madge LA, Pober JS. (2000) A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFkappaB in human endothelial cells. J. Biol. Chem. 275: 15458–15465.
Sizemore N, Leung S, Stark GR. (1999) Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol. Cell Biol. 19: 4798–4805.
Reddy SA, Huang JH, Liao WS. (1997) Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J. Biol. Chem. 272: 29167–29173.
Öberg-Welsh C, Welsh M. (1995) Cloning of BSK, A murine FRK homologe with a specific pattern of tissue distribution. Gene 152: 239–242.
Annerén C, Welsh M. (2000) Role of the Bsk/Iyk non-receptor tyrosine kinase for the control of growth and hormone production in RINm5F cells [In Process Citation]. Growth Factors 17: 233–247.
Annerén C, Reedquist KA, Bos JL, Welsh M. (2000) GTK, a Src-related tyrosine kinase, induces nerve growth factor-independent neurite outgrowth in PC12 cells through activation of the Rap1 pathway. Relationship to Shb tyrosine phosphorylation and elevated levels of focal adhesion kinase. J. Biol. Chem. 275: 29153–29161.
Öberg-Welsh C, Annerén C, Welsh M. (1998) Mutation of C-terminal tyrosine residues Y497/Y504 of the Src-family member Bsk/Iyk decreases NIH3T3 cell proliferation. Growth Factors 16: 111–124.
Sandler S, Andersson A, Hellerström C. (1987) Inhibitory effects of interleukin 1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology 121: 1424–1431.
Welsh M, Christmansson L, Karlsson T, Sandler S, Welsh N. (1999) Transgenic mice expressing Shb adaptor protein under the control of rat insulin promoter exhibit altered viability of pancreatic islet cells. Mol. Med. 5: 169–180.
Halban PA, Wollheim CB, Blondel B, Renold AE. (1980) Long-term exposure of isolated pancreatic islets to manno-heptulose: evidence for insulin degradation in the beta cell. Biochem. Pharmacol. 29: 2625–2633.
Tyrberg B, Eizirik DL, Hellerström C, Pipeleers DG, Andersson A. (1996) Human pancreatic beta-cell deoxyribonucleic acid-synthesis in islet grafts decreases with increasing organ donor age but increases in response to glucose stimulation in vitro. Endocrinology 137: 5694–5699.
Erlandsen SL, Parsons JA, Burke JP, Redick JA, Van Orden DE, Van Orden LS. (1975) A modification of the unlabeled antibody enzyme method using heterologous antisera for the light microscopic and ultrastructural localization of insulin, glucagon and growth hormone. J. Histochem. Cytochem. 23: 666–677.
Saldeen J. (2000) Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology 141: 2003–2010.
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126: 131–138.
Welsh N. (1996) Interleukin-1 beta-induced ceramide and diacylglycerol generation may lead to activation of the c-Jun NH2-terminal kinase and the transcription factor ATF2 in the insulin-producing cell line RINm5F. J. Biol. Chem. 271: 8307–8312.
Schlaepfer DD, Hauck CR, Sieg DJ. (1999) Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71: 435–478.
Avruch J. (1998) Insulin signal transduction through protein kinase cascades. Mol. Cell Biochem. 182: 31–48.
Combettes-Souverain M, Issad T. (1998) Molecular basis of insulin action. Diabetes Metab. 24: 477–489.
Mandrup-Poulsen T, Egeberg J, Nerup J, Bendtzen K, Nielsen JH, Dinarello CA. (1987) Ultrastructural studies of time-course and cellular specificity of interleukin-1 mediated islet cytotoxicity. Acta. Pathol. Microbiol. Immunol. Scand. [C] 95: 55–63.
Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. (2000) Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 256: 34–41.
Mohr S, McCormick TS, Lapetina EG. (1998) Macrophages resistant to endogenously generated nitric oxide-mediated apoptosis are hypersensitive to exogenously added nitric oxide donors: dichotomous apoptotic response independent of caspase 3 and reversal by the mitogen-activated protein kinase kinase (MEK) inhibitor PD 098059. Proc. Natl. Acad. Sci. USA 95: 5045–5050.
Birkenkamp KU, Dokter WH, Esselink MT, Jonk LJ, Kruijer W, Vellenga E. (1999) A dual function for p38 MAP kinase in hematopoietic cells: involvement in apoptosis and cell activation. Leukemia 13: 1037–1045.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331.
Takaoka A, Tanaka N, Mitani Y, et al. (1999) Protein tyrosine kinase Pyk2 mediates the Jak-dependent activation of MAPK and Stat1 in IFN-gamma, but not IFN-alpha, signaling. Embo J. 18: 2480–2488.
Xiong W, Parsons JT. (1997) Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J. Cell Biol. 139: 529–539.
Murasawa S, Matsubara H, Mori Y, et al. (2000) Angiotensin II initiates tyrosine kinase Pyk2-dependent signalings leading to activation of Rac1-mediated c-Jun NH2-terminal kinase. J. Biol. Chem. 275: 26856–26863.
Bandyopadhyay G, Sajan MP, Kanoh Y, et al. (2000) Glucose activates MAP kinase (ERK) through proline-rich tyrosine kinase-2 and the Glut1 glucose transporter. J. Biol. Chem. 275:40817–40826.
Pandey P, Avraham S, Kumar S, et al. (1999) Activation of p38 mitogen-activated protein kinase by PYK2/related adhesion focal tyrosine kinase-dependent mechanism. J. Biol. Chem. 274: 10140–10144.
Karlsson T, Kullander K, Welsh M. (1998) The Src homology 2 domain protein Shb transmits basic fibroblast growth factor-and nerve growth factor-dependent differentiation signals in PC12 cells. Cell Growth Differ. 9: 757–766.
Lu L, Annerén C, Reedquist CA, Bos JL, Welsh M. (2000) NGF-Dependent neurite outgrowth in PC12 cells overexpressing the Src homology 2-domain protein Shb requires activation of the Rap1 pathway. Exp. Cell Res. 259: 370–377.
Acknowledgments
We are grateful to Drs Helena Edlund and Ulf Ahlgren for help with generating the transgenic mice and to Ing-Britt Hallgren and Ing-Marie Mörsare for technical assistance. This work was supported by grants from the Juvenile Diabetes Foundation International, the Swedish Medical Research Council (31X-10822), the Swedish Diabetes Association, the Novo-Nordisk Foundation, and the Family Ernfors Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributed by D.F. Steiner.
Rights and permissions
About this article
Cite this article
Annerén, C., Welsh, M. Increased Cytokine-Induced Cytotoxicity of Pancreatic Islet Cells from Transgenic Mice Expressing the Src-like Tyrosine Kinase GTK. Mol Med 7, 301–310 (2001). https://doi.org/10.1007/BF03402213
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402213