Abstract
Background
Hepatitis C infection induces an acute and chronic liver inflammation that may lead to cirrhosis, liver failure, or hepatocarcinoma. Since the role of αβ T lymphocytes in hepatitis C virus (HCV) immunopathology has been analyzed extensively, we investigated the distribution and functional activation of γδ T cell subsets in chronically HCV-infected patients.
Materials and Methods
Blood samples and liver biopsies from 35 patients with compensated chronic HCV infection were compared in terms of T cell subset distribution, expression of activation markers, γδ T cell receptor (TCR) repertoire, and pattern of cytokine production. Moreover, we analyzed whether these immunological parameters were associated with other clinical observations (plasma viremia, ALT levels, Ishak index).
Results
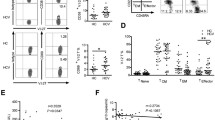
Differing from peripheral blood distribution, a specific compartmentalization of Vδ1 T cells (p < 0.001) was observed in the liver of HCV patients. These cells represented a relevant fraction of intrahepatic T lymphocytes (1.8–8.7%) and expressed the memory/effector phenotype (CD62-L− CD45-RO+CD95+). This phenotype was consistent with selective homing upon antigen recognition. Mitogenic stimulation of Vδ1+ T lymphocytes recruited in the liver revealed the T helper cell type 1 (Th1) pattern of cytokine secretion. Interestingly, the frequency of interferon-γ (IFN-γ)-producing Vδ1 T cells was associated with an higher degree of liver necroinflammation, measured by the Ishak index. Finally, the T-cell repertoire analysis revealed the absence of Vγ selection in the TCR repertoire of intrahepatic Vδ1 T cells.
Conclusions
γδ T cell distribution in the peripheral blood differs from the Vδ1 T cell subset because it is policlonally activated and recruited in the liver of chronic HCV-infected patients. During HCV-infection, this T cell subset may release Th1 cytokines and contribute to the necroinflammatory liver disease.




Similar content being viewed by others
References
Fiore G, Angarano I, Caccetta L, et al. (1996) In-situ immunophenotyping of hepatic-infiltrating cytotoxic cells in chronic active hepatitis C. Eur. J. Gastr. Hepatol. 9: 491–496.
Cohen J. (1999) The scientific challenge of hepatitis C. Science 285: 26–30.
Chisari FV, Ferrari C. (1995) Hepatitis B virus immunopathology. Springer Semin. Immunopathol. 17: 261–281.
Bianchi L. (1983) Liver biopsy interpretation in hepatitis. Phatol. Res. Pract. 178: 180–213.
Minutello MA, Pileri P, Unutmaz D, et al. (1993) Compartmentalization of T lymphocytes to the site of disease: intrahepatic CD4+ T cells specific for the protein NS4 of hepatitis C virus in patients with chronic hepatitis C. J. Exp. Med. 178: 17–25.
Nuti S, Rosa D, Valiante N, et al. (1998) Dynamics of intrahepatic lymphocytes in chronic hepatitis C: enrichment for Vα24+ T cells and rapid elimination of effector cells by apoptosis. Eur. J. Immunol. 28: 3448–3455.
Norris S, Doherty DG, Collins C, et al. (1999) Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogeneous and include Vα24-JαQ and γδ T cell receptor bearing cells. Human Immunol. 60: 20–31.
Doherty DG, Norris S, Madrigal-Estebas L, et al. (1999) The human liver contains multiple populations of NK cells, T cells, and CD3+ CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J. Immunol. 163: 2314–2321.
Gougeon ML, Boullier S, Colizzi V, Poccia F. (1999) NKR-mediated control of γδ T cell immunity to viruses. Microbes Infect. 1: 219–226.
Poccia F, Bonneville M, Lopez-Botet M, et al. (1998) Innate T cell immunity to nonpeptidic antigens and MHC class I recognition. Immunol. Today 19: 253–256.
Parker CM, Groh V, Band H, et al. (1990) Evidence for extrathymic changes in the T cell receptor γδ repertoire J. Exp. Med. 171: 1597–1612.
Hacker G, Kromer S, Falk M, Heeg K, Wagner H, Pfeffer K. (1992) Vδ1+ subset of human γδ T cells response to ligands expressed by EBV-infected Burkitt lymphoma cells and transformed B lymphocytes. J. Immunol. 149: 3984–3989.
Dechanet J, Merville P, Lim A, et al. (1999) Implication of γδ T cells in the human immune response to cytomegalovirus. J. Clin. Invest. 103: 1437–1449.
Orsini DL, Res PC, Van Laar JM, et al. (1993) A subset of Vδ1+ T cells proliferates in response to Epstein-Barr virus-transformed B cell lines in vitro. Scand. J. Immunol. 38: 335–340.
Groh V, Porcelli S, Fabbi M, et al. (1989) Human lymphocytes bearing T cell receptor γ/δ are phenotypically diverse and evenly distributed throughout the lymphoid system. J. Exp. Med. 169: 1277–1294.
Groh V, Steinle A, Bauer S, Spies T. (1998) Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279: 1737–1740.
Spada FM, Grant EP, Peters PJ, et al. (2000) Self-recognition of CD1 by γ/δ T cells: implications for innate immunity. J. Exp. Med. 191: 937–948.
Poccia F, Boullier S, Lecoeur H, et al. (1996) Pheriperal Vγ9/Vβ2 T cell deletion and anergy to non-peptidic mycobacterial antigens in asymptomatic HIV-1 infected persons. J. Immunol 157: 449–461.
Battistini L, Borsellino G, Sawicki G, et al. (1997) Phenotypic and cytokine analysis of human peripheral blood γδ T cells expressing NK. J. Immunol. 159: 3723–3730.
Sanders M, Makgoba M, Dhse D. (1988) Human naive and memory T cells. Immunol. Today 9: 195–199.
Picker L, Treer J, Ferguson D, Collins P, Buck D, Terstappen LW (1993) Control of lymphocytes recirculation in man. Differential regulation of the peripheral lymponode homing receptor L-selectin on T cells during the virgin to memory cell transition. J. Immunol. 150: 1105–1121.
Akbar AN, Terry L, Timms A, Beverley P, Janossy G. (1988) Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J. Immunol 140: 2171–2178.
Dutton RW, Bradley LM, Swain SL. (1998) T cell memory. Annu. Rev. Immunol. 16: 201–223.
Robbins PA, Evans EL, Ding AH, Warner NL, Brodsky FM. (1987) Monoclonal antibodies that distinguish between class II antigens (HLA-DP, DQ, and DR) in 14 haplotypes. Human Immunol. 18: 301–313.
Tomkinson BE, Wagner DK, Nelson DL, Sullivan JL. (1987) Activated lymphocytes during acute Epstein-Barr virus infection. J. Immunol. 139: 3802–3807.
Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. (1999) Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 163: 6236–6243.
Koziel MJ, Dudley D, Afdhal N, et al. (1995) HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J. Clin. Invest. 96: 2311–2321.
Napoli J, Bishop GA, McGuinness PH, Painter DM, McCauchghan GW. (1996) Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology 24: 759–765.
Kobalitz D, Pohl D, Pechold K. (1993) Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol. Today 14: 338–345.
Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. (1995) Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficient virus-infected individuals. J. Exp. Med. 181: 2029–2036.
Fagnoni FF, Vescovini R, Passeri G, et al. (2000) Shortage of circulating naive CD8+ T cells provides new insights on immunodeficiency in aging. Blood 95: 2860–2686.
Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst, C. (1990) Expression of murine CD1 on gastrointestinal epithelium. Science 250: 679–682.
Boullier S, Cochet M, Poccia F, Gougeon ML. (1995) CDR3-independent γδ Vδ1+ T cell expansion in the peripheral blood of HIV-1 infected persons. J. Immunol. 154: 1418–1431.
Acknowledgments
This work was supported by grants from the Current and Finalized Research Projects of the Italian Ministry of Health. C.A. is a fellow of the Institute for Infectious Diseases, L. Spallanzani. We thank M. Lupi, G. Cianca, C. Vasile, L. Bolognesi and D. Menna for clinical monitoring, organization, support and dedication to patient care. Finally we thank E. Akinde for careful reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrati, C., D’Offizi, G., Narciso, P. et al. Vδ1T Lymphocytes Expressing a Th1 Phenotype Are the Major γδ T Cell Subset Infiltrating the Liver of HCV-infected Persons. Mol Med 7, 11–19 (2001). https://doi.org/10.1007/BF03401834
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401834