Abstract
Background
Chronic diabetes causes structural changes in the retinal capillaries of nearly all patients with a disease duration of more than 15 years. Acellular occluded vessels cause hypoxia, which stimulates sight-threatening abnormal angiogenesis in 50% of all type I diabetic patients. The mechanism by which diabetes produces acellular retinal capillaries is unknown.
Materials and Methods
In this study, evidence of programmed cell death (PCD) was sought in the retinas of early diabetic rats, and the effect of nerve growth factor (NGF) on PCD and capillary morphology was evaluated.
Results
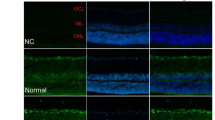
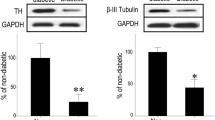
Diabetes induced PCD primarily in retinal ganglion cells (RGC) and Muller cells. This was associated with a transdifferentiation of Muller cells into an injury-associated glial fibrillary acidic protein (GFAP)-expressing phenotype, and an up-regulation of the low-affinity NGF receptor p75NGFR on both RGC and Muller cells. NGF treatment of diabetic rats prevented both early PCD in RGC and Muller cells, and the development of pericyte loss and acellular occluded capillaries.
Conclusions
These data provide new insight into the mechanism of diabetic retinal vascular damage, and suggest that NGF or other neurotrophic factors may have potential as therapeutic agents for the prevention of human diabetic retinopathy.






Similar content being viewed by others
References
Klein R, Klein BEK, Moss S, Davis MD, DeMets DL. (1984) The Wisconsin Epidemiologic Study of Diabetic Retinopathy II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 104: 520–526.
Klein R, Klein BEK, Moss S, Davis MD, DeMets DL. (1984) The Wisconsin Epidemiologic Study of Diabetic Retinopathy III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch. Ophthalmol. 102: 527–532.
Engerman RL. (1989) The pathogenesis of diabetic retinopathy. Diabetes 38: 1203–1206.
Krolewski AS, Warram JH, Rand LI, Kahn CR. (1987) Epidemiologic approach to the etiology of Type I diabetes mellitus and its complications. N. Engl. J. Med. 317: 1390–1398.
The Diabetic Retinopathy Study Research Group. (1981) Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of DRS findings. Report No. 8. Ophthalmology 88: 583–600.
Kinoshita JH. (1990) A thirty year journey in the polyol pathway. Exp. Eye Res. 50: 567–573.
DeRubertis FR, Craven PA. (1994) Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes 43: 1–8.
King GL, Shiba T, Oliver J, et al. (1994) Cellular and molecular abnormalities in the vascular endothelium of diabetes mellitus. Annu. Rev. Med. 45: 179–188.
Williamson JR, Chang K, Frangos M, et al. (1993) Hyperglycemic pseudohypoxia and diabetic complications. Diabetes 42: 801–813.
Hammes H-P, Martin S, Federlin K, et al. (1991) Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc. Natl. Acad. Sci. U.S.A. 88: 11555–11558.
Wyllie AH. (1993) Apoptosis. Br. J. Cancer 67: 205–208.
Williams GT, Smith CA. (1993) Molecular regulation of apoptosis: Genetic controls on cell death. Cell 74: 777–779.
Altar CA, Burton LE, Bennet GL, Dugich-Djordjevic M. (1991) Recombinant human nerve growth factor is biologically active and labels novel high-affinity binding sites in rat brain. Proc. Natl. Acad. Sci. U.S.A. 88: 281–285.
Kuwabara T, Cogan DG. (1960) Studies of retinal vascular patterns: normal architecture. Arch. Ophthalmol. 64: 904–911.
Engerman RL, Kern TS. (1987) Progression of diabetic retinopathy during good glycemie control. Diabetes 36: 808–812.
Gavrieli Y, Sherman Y, Ben-Sasson SA. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119: 493–501.
Okada M, Matsumura M, Ogino N, Honda Y. (1990) Muller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefes. Arch. Clin. Exp. Ophthal. 228: 467–474.
Osborne NN, Block F, Sontag KH. (1991) Reduction of ocular blood flow results in glial fibrillary acidic protein expression in rat retinal Muller cells. Vis. Neurosci. 7: 637–639.
Humphrey MF, Constable IJ, Chu Y, Wiffen S. (1993) A quantitative study of the lateral spread of Muller cell responses to retinal lesions in the rabbit. J. Comp. Neurol. 334: 545–558.
Carmignoto G, Comelli MC, Candeo P, et al. (1991) Expression of NGF receptor and NGF receptor mRNA in the developing and adult rat retina. Exp. Neurol. 111: 302–311.
Rabizadeh S, Oh J, Zhong LT, et al. (1993) Induction of apoptosis by the low-affinity NGF receptor. Science 261: 345–348.
Wolter JR. (1961) Diabetic retinopathy. Am. J. Ophthal. 51: 1123–1141.
Hori S, Mukai N. (1989) Ultrastructural lesions of retinal pericapillary Muller cells in streptozotocin-induced diabetic rats. Albrecht Von Graefes Archiv Klinische Exp. Opthalmol. 213: 1–9.
Carmignoto G, Maffei L, Candeo P, et al. (1989) Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J. Neurosci. 9: 1263–1272.
Siliprandi R, Canella R, Carmignoto G. (1993) Nerve growth factor promotes functional recovery of retinal ganglion cells after ischemia. Invest. Opthalmol. Vis. Sci. 34: 3232–3245.
Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. (1995) Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. U.S.A. 92: 905–909.
Ferrara N. (1993) Vascular endothelial growth factor. Trends Cardiovasc. Med. 3: 244–250.
Jakeman LB, Winer J, Bennet GL, Altar CA, Ferrara N. (1992) Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J. Clin. Invest. 89: 244–253.
Acknowledgments
This work was supported by National Institutes of Health Grant DK 33861-07, the Juvenile Diabetes Foundation, and Grant Hal755/1-1 from the Deutsche Forschungsgemeinschaft. We would like to thank Dr. John A. Kessler, Albert Einstein College of Medicine, for making NGF-treated animal tissue available to us.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hammes, HP., Federoff, H.J. & Brownlee, M. Nerve Growth Factor Prevents Both Neuroretinal Programmed Cell Death and Capillary Pathology in Experimental Diabetes. Mol Med 1, 527–534 (1995). https://doi.org/10.1007/BF03401589
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401589