Abstract
OBJECTIVE
This experimental study aimed to prospectively investigate the impact of combinations of prenatal and postnatal food manipulations on the metabolic profile of adult offspring.
DESIGN
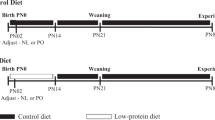
On day 12 of gestation, 67 timed pregnant rats were randomized into three nutritional groups, control: standard laboratory food; starved: 50% food restricted, FR; fat-fed: fat-rich diet, FF. Seven hundred and seventy-four (774) pups were born on day 21 and culled to 8 (4 males, 4 females) per litter to normalize rearing. Rats born to starved mothers were later subdivided, based on birthweight (BiW), into fetal growth restricted (FGR) and non-FGR. The pups were then weaned to the diet of their fostered mother until one year old. Thus, 12 groups were studied: 1.
CONTROL/CONTROL
14 rats, 2. CONTROL/FR: 12 rats, 3. CONTROL/FF: 15 rats, 4. FGR/CONTROL: 16 rats, 5. FGR/FR: 10 rats, 6. FGR/FF: 15 rats, 7. non-FGR/CONTROL: 10 rats, 8. non-FGR/FR: 17 rats, 9. non-FGR/FF: 10 rats, 10. FF/CONTROL: 15 rats, 11. FF/FR: 14 rats, and 12. FF/FF: 13 rats. During sacrifice, body weight (BW) and liver weight (LW) were measured (expressed in grams) and concentrations of serum glucose, triglycerides, HDL and NEFA were determined.
RESULTS
Postnatal food restriction, compared to control diet significantly reduced BW (p = 0.004, p = 0.036, p< 0.001, p = 0.008) and LW (p< 0.001) in all study groups. Postnatal control diet significantly increased BW in non-FGR compared to FGR rats (p = 0.027). No significant differences were detected in biochemical parameters (excluding NEFA) between FGR and non-FGR, regardless of the postnatal diet.
CONCLUSIONS
Interaction between prenatal and postnatal nutrition produces distinct metabolic profiles. Apart from BiW, prenatal diet had an important impact on the metabolic profile of the adult offspring, implying that intrauterine events should be considered in the estimation of the metabolic risk of an individual, independently of BiW.
Similar content being viewed by others
References
Dudley KJ, Sloboda DM, Connor KL, et al, 2011 Offspring of Mothers Fed a High Fat Diet Display Hepatic Cell Cycle Inhibition and Associated Changes in Gene Expression and DNA Methylation. PLoS One 7: e21662.
Weaver IC, Cervoni N, Champagne FA, et al, 2004 Epigenetic programming by maternal behavior. Nat Neurosci 7: 847–854.
Lehmann AE, Ennis K, Georgieff MK, et al, 2011 Evidence for a hyporesponsive limbic-hypothalamic-pituitary-adrenal axis following early-life repetitive hypoglycemia in adult male rats. Am J Physiol Regul Integr Comp Physiol 301: R484–490.
Alexanderson C, Stener-Victorin E, Kullberg J, et al, 2010 A single early postnatal estradiol injection affects morphology and gene expression of the ovary and parametrial adipose tissue in adult female rats. J Steroid Biochem Mol Biol 122: 82–90.
Gottrand F, 2008 Long-chain polyunsaturated fatty acids influence the immune system of infants. J Nutr 138: 1807S–1812S.
Nicolaidis S, 2008 Prenatal imprinting of postnatal specific appetites and feeding behavior. Metabolism 57: Suppl 2: 22–26.
Lukaszewski MA, Mayeur S, Fajardy I, et al, 2011 Maternal prenatal undernutrition programs adipose tissue gene expression in adult male rat offspring under high-fat diet. Am J Physiol Endocrinol Metab 301: E548–59.
Sebert S, Sharkey D, Budge H, et al, 2011 The early programming of metabolic health: is epigenetic setting the missing link? Am J Clin Nutr 94: 1953S–1958S.
Lillycrop KA, Burdge GC, 2011 Epigenetic changes in early life and future risk of obesity. Int J Obes (Lond) 2011: 72–83.
Pervanidou P, Chrousos GP, 2011 Stress and behavior: the role of nutrients with emphasis on omega-3 fatty acids World Rev Nutr Diet 102: 44–52.
Gluckman PD, Hanson MA, 2004 Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res 56: 311–317.
Woodall SM, Breier BH, Johnston BM, et al, 1996 A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. J Endocrinol 150: 231–242.
Vickers MH, Reddy S, Ikenasio BA, Breier BH, 2001 Dysregulation of the adipoinsular axis — a mechanism for the pathogenesis of hyperleptinemia and adipogenic diabetes induced by fetal programming. J Endocrinol 170: 323–332.
Franco MDC, Arruda RM, Fortes ZB, et al, 2002 Severe nutritional restriction in pregnant rats aggravates hypertension, altered vascular reactivity, and renal development in spontaneously hypertensive rats offspring. J Cardiovasc Pharmacol 39: 369–377.
Franco MDC, Akamine EH, Di Marco GS, et al, 2003 NADPH oxidase and enhanced superoxide generation in intrauterine undernourished rats: involvement of the renin-angiotensin system. Cardiovasc Res 59: 767–775.
Takemori K, Kimura T, Shirasaka N, et al, 2011 Food restriction improves glucose and lipid metabolism through Sirt1 expression: a study using a new rat model with obesity and severe hypertension. Life Sci 20: 1088–1094.
Galler JR, Tonkiss J, 1991 Prenatal protein malnutrition and maternal behavior in Sprague-Dawley rats. J Nutr 121: 762–769.
Langley SC, Browne RF, Jackson AA, 1994 Altered glucose tolerance in rats exposed to maternal low protein diets in utero. Comp Biochem Physiol Physiol 109: 223–229.
Woods LL, Ingelfinger JR, Nyengaard JR, et al, 2001 Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467.
Srinivasan M, Katewa SD, Palaniyappan A, et al, 2006 Maternal high fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in their adulthood. Am J Physiol Endocrinol Metab 291: E792–799.
Langley-Evans SC, 1996 Intrauterine programming of hypertension in the rat: nutrient interactions. Comp Biochem Physiol A Physiol 114: 327–333.
Gluckman PD, Hanson MA, Spencer HG, 2005 Predictive adaptive responses and human evolution. Trends Ecol Evol 20: 527–533.
Eleftheriades M, Creatsas G, Nicolaides K, 2006 Fetal growth restriction and postnatal development. Ann N Y Acad Sci 1092: 319–330.
Entringer S, Epel ES, Kumsta R, et al, 2011 Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci USA 108: E513–518.
Pervanidou P, Chrousos GP, 2012 Metabolic consequences of stress during childhood and adolescence. Metabolism 61: 611–619.
Weir GC, Laybutt DR, Kaneto H, et al, 2001 Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes 50: Suppl 1: 154–159.
Turan OM, Turan S, Gungor S, et al, 2008 Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet Gynecol 32: 160–167.
Tosh DN, Fu Q, Callaway CW, et al, 2010 Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth Am J Physiol Gastrointest Liver Physiol 299: G1023–1029.
Desai M, Gayle D, Babu J, et al, 2005 Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol 288: R91–R96.
Nobili V, Alisi A, Panera N, et al, 2008 Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev 6: 241–247.
Jonas JC, Sharma A, Hasenkamp W, et al, 1999 Chronic hyperglycemia triggers loss of pancreatic β-cell differentiation in an animal model of diabetes. J Biol Chem 274: 14112–14121.
de Souza CJ, Capotorto JV, Cornell-Kennon S, et al, 2000 The beta-cell dysfunction in 48-hour glucose infused rats is not a consequence of elevated plasma lipid or islet triglyceride levels. Metabolism 49: 755–769.
Ling PR, Bistrian BR, 2009 Comparison of the effects of food versus protein restriction on selected nutritional and inflammatory markers in rats. Metabolism 58: 835–842.
Zaman MQ, Leray V, Le Bloch J, et al, 2011 Lipid profile and insulin sensitivity in rats fed with high-fat or high-fructose diets. Br JNutr 106: Suppl 1: 206–210.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eleftheriades, M., Pervanidou, P., Vafaei, H. et al. Metabolic profiles of adult Wistar rats in relation to prenatal and postnatal nutritional manipulation: The role of birthweight. Hormones 13, 268–279 (2014). https://doi.org/10.1007/BF03401341
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401341