Abstract
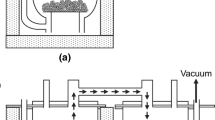
Production of anhydrous zirconium tetrachloride by direct chlorination of a zirconium oxide-corbon mixture in a silica-brick-lined chlorinator is described. Theory and thermodynamics of reactions are discussed. A pilot-model chlorinator and full-scale production equipment are described and operating data are included.
Similar content being viewed by others
References
W. J. Kroll, A. W. Schlechten, and L. A. Yerkes: Ductile Zirconium from Zircon Sand. Trans. Electrochem. Soc. (1946) 89, pp. 263–276.
W. J. Kroll, A. W. Schlechten, W. R. Carmody, L. A. Yerkes, H. P. Holmes, and H. L. Gilbert: Recent Progress in the Metallurgy of Malleable Zirconium. Trans. Electrochem. Soc. (1947) 92, pp. 99–113.
W. J. Kroll, C. T. Anderson, H. P. Holmes, L. A. Yerkes, and H. L. Gilbert: Large Scale Laboratory Production of Ductile Zirconium. Journal Electrochem. Soc. (1948) 94, No. 1, pp. 1–20.
W. J. Kroll, W. W. Stephens, and H. P. Holmes: Production of Malleable Zirconium on a Pilot-Plant Scale. Trans. AIME (1950) 188, pp. 1445–1453; Journal of Metals (December 1950).
W. J. Kroll, W. W. Stephens, and J. P. Walsted: Graphite-Rod Hairpin-Resistor Reduction Furnace for High Temperatures. Trans. AIME (1950) 188, pp. 1394–1395; Journal of Metals (November 1950).
All data for CO, CO2, O2, and Cl2 as well as ΔH298, 16 for ZrO2(c) and ZrCl4(c) from Selected Values of Chemical Thermodynamic Properties. Nat. Bur. Standards.
H°T-H°298,16 and S°T-S°298,16 for Zr(c) and ZrO2(c) from Coughlin and King: Journal Amer. Chem. Soc. 72, pp. 2262–2265.
H°T-H°298,16 and S°T-S°298,16 for ZrCl4(g) calculated by K. K. Kelley, Chief, Minerals Thermodynamics Branch, Region III, Bureau of Mines, Berkeley, Calif.
H. A. Doerner, and W. F. Holbrook: Chlorination of Magnesia. U. S. Bur. Mines R. I. 3833 (December 1945) p. 12.
Ford H. McBerty: Anhydrous Chlorides Manufacture. F.I.A.T. Final Report No. 774 (1946) pp. 15–18.
Author information
Authors and Affiliations
Additional information
Discussion on this paper, TP B332D, may be sent, 2 copies, to AIME by Sept. 1, 1952. Manuscript, Jan. 23, 1952. New York Meeting, February 1952.
Papers by authors of the U. S. Bureau of Mines staff are not subject to copyright.
Rights and permissions
About this article
Cite this article
Stephens, W.W., Gilbert, H.L. Chlorination of Zirconium Oxide. JOM 4, 733–737 (1952). https://doi.org/10.1007/BF03398133
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03398133