Abstract
Processes employing direct oxidation under an over-pressure of air or oxygen in an aqueous sulphuric acid medium have been developed in the Sherritt Gordon Laboratories for iron, nickel, cobalt, zinc and lead sulphide concentrates. This study has recently also been extended to chalcocite, Cu2S, concentrates. The rising interest in processes employing direct aqueous oxidation is stimulated by the fact that elemental sulphur can be produced as a by-product rather than sulphur dioxide or sulphuric acid.
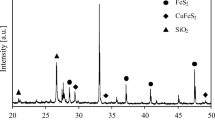
The present paper outlines a process which features the direct pressure oxidation of the most abundant copper sulphide mineral, chalcopyrite, CuFeS2. The optimum conditions for a practical pressure leaching step have now been developed in the laboratory which results in the production of copper sulphate solution suitable for copper winninq by electrolysis, hydrogen reduction, solvent extraction combined with electrolysis, or other means. The leach residue yields pure elemental sulphur by-product. Copper and elemental sulphur recoveries of 98 and 85% respectively have been recorded. The fastest oxidation rate, corresponding to a leach retention time of 2.5 hr, was obtained when the copper concentrate was ground to 99.5% — 325 mesh, when a 50% stoichiometric excess of concentrate over the amount of available sulphuric acid for copper was used and when the oxygen partial pressure and temperature were maintained at 500 psi and 240°F, respectively. In an idealized form, the pressure leaching reaction can be expressed as follows:—CuFeS2 + H2SO4 + 1 1/402 + 1/2H2O → CuSO4 + Fe(OH)3 + 2S°
After separation of the copper sulphate solution by filtration, elemental sulphur and excess concentrate ore recovered from the iron oxide tailing by flotation. The tailing, containing iron oxide and insolubles, is rejected. The elemental sulphur is separated from the concentrate by hot filtration, solvent extraction, distillation, or other means, and the unleached chalcopyrite is recycled to the leaching step.
Similar content being viewed by others
Reference
H. Veltman, S. Pellegrini, and V. N. Mackiw: Direct Acid Pressure of Chalcocite Concentrate, Journal of Metals, vol. 19, No. 2, pp. 21–25, 1967.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vizsolyi, A., Veltman, H., Warren, I.H. et al. Copper and elemental sulphur from chalcopyrite by pressure leaching. JOM 19, 52–59 (1967). https://doi.org/10.1007/BF03378656
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03378656