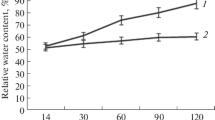
Abstract
Plantago ovata is commercially grown in India for its dietary fibre. The crop is affected by downy mildew caused by Peronospora plantaginis Underwood leading to severe yield loss. A study was undertaken to assess the impact of downy mildew on host photosynthesis under field conditions. The results showed that primary photosynthetic pigments (total chlorophyll) were reduced by 34.74% and 62.11% in slight and severe chlorotic leaves, respectively, compared to healthy leaves. Net photosynthetic rate (Pn) was also significantly reduced in diseased leaves. The diurnal photosynthesis measurement showed that peak Pn in the diseased leaves was short lived as compared to healthy leaves. Infection caused an increase in dark respiration (Rd) and intracellular CO2 concentration (Ci), while stomatal conductance (gs) was similar to that of healthy leaves during peak photosynthetic period of the day. Infection caused a reduction in soluble sugar content accompanied by an increase in leaf starch content. Vitality index (Fv/Fm) of the diseased leaves was reduced by 24.39% in slight chlorotic and 44.90% in severe chlorotic leaves as compared to healthy leaves. Further, quantum yield of photosystem (PS) II (FPSII) showed severe reduction, which was correlated with the Pn. Our study suggests that carbon assimilation in the diseased leaves is mainly limited by PSII function. Disease induced reduction in chlorophyll content appears to be one of the causes for reduction in PSII yield. Increase in starch accumulation in infected leaves appears to be a cause for reduction in photosynthesis in infected leaves.
Zusammenfassung
Das Wegerichgewächs (Fam. Plantaginaceae) Plantago ovata wird in wegen seiner als ballaststoffreiches Nahrungsmittel verwendeten Samenschalen (Isabgol) angebaut. Der Befall der Pflanze mit dem Erreger des Falschen Mehltaus, Peronospora plantaginis Underwood, kann zu bedeutenden Ertragsverlusten führen. Im Feld wurde daher der Einfluss des Erregers auf die Photosynthese des Wirtes untersucht. Der Gesamtchlorophyllgehalt war in leicht und stark chlorotischen Blättern um 34,74 bzw. 62,11% gegenüber nicht befallenen Blättern vermindert. Die Nettophotosyntheserate (Pn) erkrankter Blätter war ebenfalls signifikant reduziert. Messungen diurnaler Veränderungen der Phytosynthese zeigten, dass der Pn-Peak befallener Blätter im Vergleich zu gesunden relativ kurzlebig war. Ein Befall erhöhte sowohl die Dunkelatmung (Rd) als auch die intrazelluläre CO2-Konzentration (Ci), während die stomatäre Leitfähigkeit (gs) in der Periode maximaler photosynthetischer Aktivität keine Unterschiede zu nicht befallenen Blättern aufwies. Befallsbedingt sank der Gehalt löslicher Zucker, während der Stärkegehalt der Blätter stieg. Der Vitalitätsindex (Fv/Fm) befallener Blätter sank gegenüber der Gesundvariante um 24,39% in schwach chlorotischen und um 44,90% in stark chlorotischen Blättern. Die Quantenausbeute des Photosystems (PS) II (FPSII) war in Kor- relation mit Pn stark vermindert. Die Untersuchung deutet darauf hin, dass die photosynthetische Kohlenstoffassimilation befallener Blätter vor allem durch die Funktion des PS II begrenzt wird. Der befallsbedingt verminderte Gehalt an Gesamtchlorophyll scheint die Ausbeute des PS II negativ zu beeinflussen. Die erhöhte Stärkespeicherung erkrankter Blätter scheint sich wiederum negativ auf die Photosyntheseleistung auszuwirken.
Similar content being viewed by others
References
Anonymous, 2007: Health claims: soluble fiber from certain foods and risk of coronary heart disease (CHD) [electronic version], In: Code of Federal Regulations, pp. 442–446. Food and Drug Administration Department of Health and Human Services, Washington, D.C., USA. Retrieved November 28, 2007 from, http://www.access.gpo.gov/nara/cfr/waisidx_07/21cfr101_07.html.
Bassanezi, R.B., L. Amorim, A.B. Filho, R.D. Berger, 2002: Gas exchange and emission of chlorophyll fluorescence during the monocycle of rust, angular leaf spot and anthracnose on bean leaves as a function of their trophic characteristics. J. Phytopathol. 150, 1–11.
Boote, K.J., J.W. Jones, G.H. Smerage, C.S. Barfield, R.D. Berger, 1980: Photosynthesis of peanut canopies as affected by leaf spot and artificial defoliation. Agron. J. 72, 247–252.
Cheng, L-S., L. Cheng, 2004: CO2 assimilation, carbohydrate metabolism, xanthophylls cycle, and the antioxidant system of ‘Honeycrisp’ apple leaves with zonal chlorosis. J. Am. Soc. Hortic. Sci. 129, 729–737.
Guo, D-P., Y-P. Guo, J-P. Zhao, H. Liu, Y. Peng, Q-M. Wang, J-S. Chen, G-Z. Rao, 2005: Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var. tsatsai) after turnip mosaic virus infection. Plant Sci. 168, 57–63.
Habermann, G., E.C. Machado, J.D. Rodrigues, C.L. Medina, 2003: CO2 assimilation, photosynthetic light response curves, and water relations of ‘Pera’ sweet orange plants infected with Xylella fastidiosa. Braz. J. Plant Physiol. 15, 79–87.
Hiscox, J.D., G.F. Israelstam, 1979: A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 57, 1332–1334.
Ingold, C.T., 1960: Dispersal by air and water — the take-off. In: J.G. Horsfall, A.E. Dimond (eds.): Plant Disease, an Advanced Treatise Vol. 3, pp. 137–168. Academic Press, New York.
Ingram, D.S., 1981: Physiology and biochemistry of hostparasite interaction. In: D.M. Spencer (ed.): The Downy Mildews, pp. 143–163. Academic Press, London.
Lindenthal, M., U. Steiner, H.-W. Dehne, E.-C. Oerke, 2005: Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology 95, 233–240.
Livne, A., 1964: Photosynthesis in healthy and rust affected plants. Plant Physiol. 39, 614–621.
Lopes, D.B., R.D. Berger, 2001: The effects of rust and anthracnose on the photosynthetic competence of diseased bean leaves. Phytopathology 91, 212–220.
Mahadevan, A., R. Sridhar, 1982: Methods in Physiological Plant Pathology, 2nd edition. Sivakami Publications, Chennai, India.
Mandal, K., N.A. Gajbhiye, S. Maiti, 2007: Fungicidal management of downy mildew of isabgol simulating farmers’ field-conditions. Australas. Plant Pathol. 36, 186–190.
Maxwell, K., G.N. Johnson, 2000: Chlorophyll fluorescence — a practical guide. J. Exp. Bot. 51, 659–668.
Miller, G.L., 1972: Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428.
Moll, S., P. Serranoo, C. Boyle, 1995: In vivo chlorophyll fluorescence in rust-infected bean plants. Angew. Bot. 69, 163–168.
Moriondo, M., S. Orlandini, A. Giuntoli, M. Bindi, 2005: The effect of downy and powdery mildew on grapevine (Vitis vinifera L.) leaf gas exchange. J. Phytopathol. 153, 350–357.
Raggi, V., 1978: The CO2 compensation point, photosynthesis and respiration in rust infected bean leaves. Physiol. Plant Pathol. 13, 135–139.
Scholes, J.D., S.A. Rolfe, 1995: How do biotrophic pathogens affect the photosynthetic metabolism of their host? Asp. Appl. Biol. 42, 91–99.
Sharma, M.P., D. Rajpurohit, 2004: Biochemical alterations in isabgol leaves in response to fungicidal control of downy mildew. J. Mycol. Plant Pathol. 34, 130–132.
Shtienberg, D., 1992: Effects of foliar diseases on gas exchange processes: a comparative study. Phytopathology 82, 760–765.
Srinivasan, N., R. Jeyarajan, 1977: Grape downy mildew in India. IV. Effect of infection on sugars content and respiration in leaves. Indian J. Hortic. 34, 209–214.
Thornton, J.D., R.C. Cooke, 1974: Changes in respiration, chlorophyll content and soluble carbohydrates of detached cabbage cotyledons following infection with Peronospora parasitica (Pers. ex Fr.) Physiol. Plant Pathol. 4, 117–125.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandal, K., Saravanan, R., Maiti, S. et al. Effect of downy mildew disease on photosynthesis and chlorophyll fluorescence in Plantago ovata Forsk. J Plant Dis Prot 116, 164–168 (2009). https://doi.org/10.1007/BF03356305
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03356305