Abstract
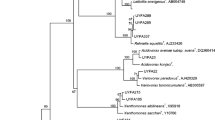
Detection and identification of ice-nucleation active (INA) bacteria, was carried out in several independent investigations from diseased willow plants in different regions in Sweden and Estonia. Many of these bacteria, alone or together, cause serious bacterial disease problems in willow (Salix spp.) plants in combination with frost leading to dieback in plantations for energy forestry purposes. Methods used for identification were Biolog® MicroPlates, biochemical tests including growth in different media and pathogenic tests, designing and using selective INA primers, and 16S rRNA gene analyses. The taxonomic tools, especially phyloge-netic analysis derived from 16S rRNA gene sequences, clearly distinguished many bacteria. The identified strains from willows (20 clones) belonged to at least eight different genera and 12 species showing variable levels of aggressiveness and ice-nucleation activity under laboratory and greenhouse conditions. Diseased willows were found associated with the presence of Agrobacterium tumefaciens, Bacillus spp., Clavi-bacter spp., Erwinia rhapontici, Frigoribacterium faeni, Pseudomonas brenneri, P. fluorescens, P. frederiksbergensis, P. graminis, P. syringae, P. veronii, Sphingobacterium/ Pedobacter, Sphingomonas/non-fluorescent P. fluorescens (different biotypes), Xanthomonas campestris and related species.
Zusammenfassung
Eiskristalle bildende (ice-nucleation active, INA) Bakterien, die Schäden an Weiden (Salix spp.) bei Frost im Spätherbst, Winter und Frühjahr verursachen können, wurden mittels BIOLOG® Mikroplatten, biochemischer Tests einschlieÞlich Wachstumsuntersuchungen in verschiedenen Medien und Pathogenitätstests, der Herstellung und Verwendung selektiver INA-Primer, und 16S rRNA-Analysen nachgewiesen und identifiziert. Die identifizierten Bakterienisolate konnten mindestens acht Genera und 12 Arten zugeordnet werden; sie zeigten Unterschiede in der Aggressivität und dem Vermögen zur Eiskristallbildung unter Labor- und Freilandbedingungen. Mit Hilfe taxonomischer Werkzeuge, insbesondere auf 16S rRNA-Sequenzen basierenden phylogenetischen Analysen, konnten viele Bakterien voneinander unterschieden werden, die während mehrerer unabhängig voneinander durchgeführter Untersuchungen aus erkrankten Weidenbäumen in verschiedenen Regionen Schwedens und Estlands isoliert worden waren. Gemeinschaften dieser Bakterien scheinen ein ernstes Krankheitsproblem bis hin zum Absterben bei Weiden zu verursachen, die zur Energiegewinnung in Plantagen angebaut werden. Erkrankte Weiden waren mit Bakterien der Gattungen oder Arten Agrobacterium tumefaciens, Bacillus spp., Clavibacter spp., Erwinia rhapontici, Frigoribacterium faeni, Pseudomonas brenneri, P. fluorescens, P. frederiksbergensis, P. graminis, P. syringae, P. veronii, Sphingobacterium/Pedobacter, Sphingomonas//nicht-fluoreszierende P. fluorescens (verschiedene Biotypen), Xanthomonas campestris und anderen verwandten Arten assoziiert.
Similar content being viewed by others
Literature
Andersen, S.M., K. Johnsen, J. Jorensen, P. Nilsen, C.S. Jacobsen, 2000: Pseudomonas frederiksbergensis sp. nov., isolated from soil at a coal gasification site. Int. J. Syst. Evol. Microbiol. 50, 1957–1964.
Abe, K., S. Watabe, Y. Emori, M. Watanabe, S. Arai, 1989: An ice nucleation active gene of Erwinia ananas: Sequence similarity to those of Pseudomonas species and regions required for ice nucleation activity. Febs Lett. 258, 297–300.
Hensyl, W.R., ed. 1993: Bergey’s Manual of Determinative Bacteriology, ninth edition. Williams and Wilkins, Baltimore, MD, USA.
Boelema, B.H., 1972: Bacterial blight (Pseudomonas pisi Sack-ett) of peas in South Africa with a special reference to frost as predisposing factor. Meded. Landbouwhogesch. Wageningen 72, 1–87.
Borrel, N., S.G. Acinas, M.J. Figueras, A.J. Martinez-Muricia, 1997: Identification of Aeromonas clinical isolates by restriction fragment length polymorphism of Pcr-amplified 16S rRna genes. J. Clin. Microbiol. 35, 1671–1674.
Burke, M.J., L.A. Gusta, H.A. Quamme, C.J. Weiser, P.H. Li, 1976: Freezing and injury to plants. Annu. Rev. Plant Physiol. 27, 507–528.
Cambours, M.A., 2004: Ice nucleation-active and pathogenic bacteria in Swedish and Estonian Salix short-rotation forestry plantations suffering from frost-related dieback. Licentiate thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden. IsbN 91-576-6637-7.
Cambours, M.A., P. Nejad, U. Granhall, M. Ramstedt, 2005: Frost-related dieback of willows, comparison of epiphytic and endophytic bacteria of different Salix clones, with emphasis on ice-nucleation activity, pathogenic properties and seasonal variation. Biomass Bioenerg. 28, 15–27.
De Kam, M., 1978: Xanthomonas populi subs. salicis, cause of bacterial canker in Salix dasyclada. Eur. J. Forest Pathol. 8, 334–337.
Destefano, S.A.L., I.M.G. Almeida, N.J. Rodrigues, M. Ferreira, D.M. Balani, 2003: Differentiation of Xanthomonas species pathogenic to sugarcane by PCR-RFLP analysis. Eur. J. Plant Pathol. 109, 283–288.
Dong, A.-R., X.-Y. Zhang, Y.-T. Wang, Q.-Z. Zheng, J. Li, 2001: Pathogenic and physiological mechanisms of poplar ice-nu-cleation active bacterial canker. J. Forestr. Res. 12, 253–256.
Dowson, W.J., 1957: Plant Diseases due to Bacteria, second edition. Cambridge University Press, Cambridge, United Kingdom.
Felsenstein, J., 1993: PHYLIP (Phylogeny Inference Package) version 3.5c. Distributed by the author. Department of Genetics, University of Washington, Seattle, WA, USA.
Geider, K, W.S. Kim, S. Jock, M. Hildebrand, J.P. Paulin, S.L. Rhim, S. Iwahori, H. Gemma, A.D. Webster, A.G. White, 2002: Characterization of pathogenic bacteria from Korea and Japan causing Asian pear blight. In: Proceeding of the international symposium on Asian pears commemorating the 100th anniversary of Nijisseiki pear, Kurayoshi, Tottori, Japan. Acta Hort. 587, 631–638.
Green, R., G. Warren, 1985: Physical and functional repetition in bacterial ice nucleation gene. Nature 317, 645–648.
Gross, D.C., Y.S. Cody, E.L. Proebsting Jr., G.K. Radamaker, R.A. Spotts, 1983: Distribution, population dynamics, and characteristics of ice nucleation-active bacteria in deciduous fruit tree orchards. Appl. Environ. Microbiol. 46, 1370–1379.
Holmes, B.R., J. Owen, A. Evans, H. Malnick, W.R. Wilcox, 1977: Pseudomonas paucimobilis, a new species isolated from human clinical specimens, and other sources. Int. J. Bacteriol. 27, 133–146.
Hottinger, H., P. Niederberger, D. Pridmore, U. Staeger-Roose, 1991: Additives for promoting ice nucleation in ingestible biological materials. European patent no. 0424771A1.
Kampfer, P., F.A. Rainey, M.A. Andersson, L.E. Nurmiaho Lassila, U. Ulrych, H.J. Busse, N. Weiss, R. Mikkola, M. Salkinoja-Salonen, 2000: Frigoribacterium faeni gen. nov., sp. nov., a novel psychrophilic genus of the family Microbacteriaceae. Int. J. Syst.Evol. Microbiol. 50, 355–363.
Kerkoud, M., C. Manceau, J.P. Paulin, 2002: Rapid diagnosis of Pseudomonas syringae pv. papulans, the causal agent of blister spot of apple, by polymerase chain reaction using specifically designed hrpL gene primers. Phytopathology 92, 1077–1083.
Koike, S.T., 2001: Xanthomonas leaf spot of catnip: a new disease caused by a pathovar of Xanthomonas campestris. Plant Dis. 85, 1157–1159.
Kosako, Y., E. Yabuuchi, T. Naka, N. Fujiwara, K. Kobayashi, 2000: Proposal of Sphingomonadaceae fam. nov., consisting of Sphingomonas Yabuuchi et al. 1990, Erythobacter Shiba and Shimidi 1982, Erythomicrobium Yurkov et al. 1994, Porphyrobacter Fuerst et al. 1993, Zymomonas Kluyver and van Niel 1936, and Sandaracinobacter Yurkov et al. 1997, with the type genus Sphingomonas Yabuuchi et al. 1990. Microbiol. Immunol. 44, 563–575.
Kozinska, A., M.J. Figueras, M.R. Chacon, L. Soler, 2002: Phenotypic characteristics and pathogenicity of Aeromonas genomospecies isolated from common carp (Cyprinus carpino L.) J. Appl. Microbiol. 93, 1034.
Lansade, J.A., 1946: Researchers sur le chancre de peuplier en France. Ann. Epiphyt. 12, 23–31.
Lelliott, R.A., R.S. Dickey, 1984: Erwinia. In: N.R. Krieg, J.G. Holt (eds.): Bergey’s Manual of Systematic Bacteriology, vol. 1, pp. 469–476. Williams and Wilkins, Baltimore, MD, USA.
Lindow, S.E., 1983: Methods of preventing frost injury caused by epiphytic ice nucleation active bacteria. Plant Dis. 67, 327–333.
Mcdoland, J.G., E. Wong, 2001: Use of a monoclonal antibody and genomic fingerprinting by repetitive-sequence-based polymerase chain reaction to identify Xanthomonas populi pathovars. Can J. Plant Pathol. 23, 47–51.
Mcivor, I., S. Sivakumaran, S. Gardiner, P. Jameson, S. Lang, 2002: What causes the vascular nodules in fruit of some “Gala” x ”Splendour” cultivars? Poster presented at the Xxvith International Horticultural Congress, held in Toronto, Canada, August 11-17, 2002.
Nejad, P., P.A. Johnson, 2000: Endophytic bacteria induce growth promotion and wilt disease suppression in oilseed rape and tomato. Biol. Control 18, 208–215.
Nejad, P., M. Ramstedt, U. Granhall, 2002: Synergistic effect between frost damage and bacterial infection in Salix plants. Poster presented at International Poplar Symposium III on Basic and Applied Aspects of Poplar and Willow Biology; held in Uppsala, Sweden, August 26-29, 2002, Access: <http://www.forestresearch.co.nz/Pdf/07.56Nejadetal.pdf>.
Nejad, P., M. Ramstedt, U. Granhall, M.A. Cambours, S. Roos, 2003: Biochemical characterization and identification of Ice-Nucleation-Active (INA willow pathogens by means of Biolog MicroPlates, InA gene primers and PcR based-16S rRna analyses. Poster presented at the 8th International Congress of Plant Pathology (IcpP), held in Christchurch, New Zealand, February 2–7, 2003. Access, <http://www. forestresearch.co.nz/Pdf/00.02Nejadetal.pdf>.
Nejad, P., M. Ramstedt, U. Granhall, 2004: Pathogenic ice-nu-cleation active bacteria in willows for short rotation forestry. Forest Pathol. 34, 369–381.
Nejad, P., U. Granhall, M. Ramstedt, 2006: Factors influencing pathogenic Ice-Nucleation Active (INA bacteria isolated from Salix plants, soil and litter. J. Agric. Technol. 1, 207–222.
Page, R.D., M. Treeview, 1996: an application to display phylogenetic trees on personal computers. Comp. Appl. Biosci. 12, 357–358.
Olien, C.R., 1967: Freezing stresses and survival. Annu. Rev. Plant Physiol. 18, 387–408.
Orser, C., B.J. Staskawicz, J. Loper, N.J. Panopoulos, D. Dahlbeck, S.E. Lindow, M.N. Schroth, 1983: Cloning of genes involved in bacterial ice-nucleation and fluorescent pig-ment/siderophore production. In: A. Puhler (ed.): Molecular Genetics of the Bacteria-Plant Interaction, pp. 353–361. Springer-Verlag, Berlin.
Panagopoulos, C.G., J.E. Grosse, 1964: Frost injury as a predisposing factor in blossom blight of pear caused by Pseudomonas syringae van Hall. Nature 202, 1352.
Parrott, C.C., 1993: Recombinant DnA to protect crops. Woodrow Wilson Biology Institute, Princeton, NJ, USA.
Ramstedt, M., B. Åström, A.H. Von Fircks, 1994: Dieback of poplar and willow caused by Pseudomonas syringae in combination with freezing stress. Eur. J. Forest Pathol. 24, 305–315.
Rosello, M., V.S. Garcia, P. Liop, M.T. Gorris, V. Donat, J. Penalver, M.M. Lopez, A. Tarin, R. Chartier, J.P. Paulin, L. Gardan, C. Hale, R. Mitchell, 2002: Characterization of an Erwinia sp. isolated from necrotic pear blossoms in Valencia, Spain. Acta Hort. 590, 139–142.
Sahin, F., R. Kotan, P.A. Abbasi, S.A. Miller, 2003: Phenotypic and genotypic characterization of Xanthomonas campestris pv. zinniae strains. Eur. J. Plant Pathol. 109, 165–172.
Sabet, K.A., 1953: Studies on the bacterial die-back and canker disease of poplar III: Freezing in relation to the disease. Ann. Appl. Biol. 40, 645–650.
Schaad, N.W., 1988: Laboratory guide for identification of plant pathogenic bacteria. American Phytopathological Society, St. Paul, MN, USA.
Si-Wen, J., E. Giuffra, L. Andersson, X. Yuan-Zhu, 2001: Molecular phylogenetic relationship between six Chinese native pig breeds and three Swedish pig breeds from mitochondrial DNA. Acta Genet. Sin. 28, 1120–1128.
Sly, L.I., 1985: Emendation of the genus Blastobacter zavarani 1961 and description of Blastobacter natatorius sp. nov. Int. J. Syst. Bacteriol. 35, 40–45.
Suslow, T.V., M.N. Schroth, 1982: Role of deleterious rhizo-bacteria as minor pathogens in reducing crop growth. Phytopathology 72, 111–15.
Takeuchi, M., A. Yokota, 1992: Proposals of Sphingobacterium faecium sp. nov., Sphingobacterium piscium sp. nov., Sphin-gobacterium heparinum comb. nov., Sphingobacterium thalpophilum comb. nov. and two genospecies of the genus Sphingobacterium and synonymy of Flavobacterium yabuu-chiae and Sphingobacterium spiritivorum. J. Gen. Appl. Microbiol. 38, 465–482.
Takeuchi, M., F. Kawai, Y. Shimada, A. Yokota, 1993: Taxonomic study of polyethylene glycol-utilizing bacteria: emended description of the genus Sphingomonas and new descriptions of Sphingomonas macrogoltabidus sp. nov., Sphingomonas sanguis sp. nov. and Sphingomonas terrae sp. nov. Syst. Appl. Microbiol. 16, 227–238.
Thompson, J.D., D.G. Higgins, T.J. Gibson, 1994: Clustal W: improving the sensitivity of progressive multiple sequence alignment through weighting, positions-specific gap penalties and weight matrix choice. Nucl. Acids Res. 22, 4673–4680.
Ventura, M., R. Zink, 2002: Specific identification and molecular typing analysis of Lactobacillus johnsonii by using Pcr-based methods and pulsed-field gel electrophoresis. FEMS Microbiol. Lett. 217, 141–154.
Wilson, M., S.S. Hirano, S.E. Lindow, 1999: Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl. Environ. Microbiol. 65, 1435–1443.
Warren, G., L. Corotto, 1989: The consensus sequence of ice nucleation proteins from Erwinia herbicola, Pseudomonas fluorescens and Pseudomonas syringae. Gene 85, 239–242.
Warren, G., L. Corotto, P. Wolber, 1986: Conserved repeats in diverged ice nucleation structural genes from two species of Pseudomonas. Nucl. Acids Res. 14, 8047–8060.
Woese, C.R., R. Gutell, R. Gupta, H.F. Noller, 1983: Detailed higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol. Rev. 47, 621–669.
Yabuuchi, E., L. Yano, H. Oyaizu, Y. Hashimoto, T. Ezaki, H. Yamamoto, 1990: Proposals of Sphingomonas paucimobilis gen. nov., and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genome species of the genus Sphingomonas. Microbiol. Immunol. 34, 99–119.
Yabuuchi, E., Y. Kosako, T. Naka, S. Suzuki., I. Yano, 1999: Proposal of Shingomonas suberifaciens (Van Bruggen, Jochimsen and Brown 1990) comb. nov., Sphingomonas natatoria (Sly 1985) comb. nov., Sphingomonas ursincola (Yurkov et al. 1997) comb. nov., emendation of the genus Sphingomonas. Microbiol. Immunol. 43, 339–349.
Yabuuchi, E., Y. Kosako, N. Fujiwara, T. Naka, I. Matsunaga, H. Ogura, K. Kobayashi, 2002: Emendation of the genus Sphingomonas Yabuuchi et al. 1990 and junior objective synonym of the species of three genera, Sphingobium, Novosph-ingobium and Sphingopyxis, in conjunction with Blastomonas ursincola. Int. J. Syst. Evol. Microbiol. 52, 1485–1496.
Yamamoto, A., I. Yano, M. Masui, E. Yabuuchi, 1978: Isolation of a novel sphingoglycolipid containing glucuronic acid and 2-hydroxy fatty acid from Flavobacterium devorans ATCC 10829. J. Biochem. 83, 1213–1216.
Zhao, J., C.S. Orser, 1990: Conserved repetition in the ice nucleation gene inaX from Xanthomonas campestris pv. translucens. Genetics 223, 163–166.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nejad, P., Ramstedt, M., Granhall, U. et al. Biochemical characterization and identification of ice-nucleation-active (INA) willow pathogens by means of BIOLOG® MicroPlate, INA gene primers and PCR-based 16S rRNA-gene analyses. J Plant Dis Prot 113, 97–106 (2006). https://doi.org/10.1007/BF03356165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03356165