Abstract
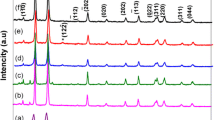
Modulation of band energies through size control offers new ways to control photoresponse and photoconversion efficiency of the solar cell. The P-type semiconductor of copper oxide is an important functional material used for photovoltaic cells. CuO is attractive as a selective solar absorber since it has high solar absorbance and a low thermal emittance. The present work describes the synthesis and characterization of semiconducting CuO nanoparticles via one-step, solid-state reaction in the presence of Polyethylene glycol 400 as size controlling agent for the preparation of CuO nanoparticles at different temperatures. Solid-state mechanochemical processing, which is not only a physical size reduction process in conventional milling but also a chemical reaction, is mechanically activated at the nanoscale during grinding. The present method is a simple and efficient method of preparing nanoparticles with high yield at low cost. The structural and chemical composition of the nanoparticles were analyzed by X-ray diffraction, field emission scanning electron microscopy and energy-dispersive spectrometer, respectively. Optical properties and band gap of CuO nanoparticles were studied by UV-Vis spectroscopy. These results showed that the band gap energy decreased with increase of annealing temperature, which can be attributed to the improvement in grain size of the samples.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Sambandam Anandan, Sol. Energy Mater. Sol. Cells 91, 843 (2007). http://dx.doi.org/10.1016/j.solmat.2006.11.017
A. Henglein, Chem. Rev. 89, 1861 (1989). http://dx.doi.org/10.1021/cr00098a010
A. Agfeldt and M. Gratzel, Chem. Rev. 95, 49 (1995). http://dx.doi.org/10.1021/cr00033a003
Hui Wang, Jin-Zhong Xu, Jun-Jie Zhu and Hong-Yuan Chen, J. Cryst. Growth 244, 88 (2002). http://dx.doi.org/10.1016/S0022-0248(02)01571-3
Julia Hambrock, Ralf Becker, Alexander Birkner, Jurij Weiß and Roland A. Fischer, Chem. Commun. 68–69 (2002). http://dx.doi.org/10.1039/b108797e
Bong Kyun Park, Sunho Jeong, Dongjo Kim, Jooho Moon, Soonkwon Lim and Jang Sub Kim, J. Colloid Interface Sci. 311, 417 (2007). http://dx.doi.org/10.1016/j.jcis.2007.03.039
Masoud Salavati-Niasari and Fatemeh Davar, Mater. Lett. 63, 441 (2009). http://dx.doi.org/10.1016/j.matlet.2008.11.023
Chih-Hung Lo and Tsing-Tshih Tsung, J. Vac. Sci. Technol. B 23, 2394 (2005). http://dx.doi.org/10.1116/1.2122787
Junwu Zhu, Haiqun Chen, Hongbo Liu, Xujie Yang, Lu. Lude and Xin Wang, Mater. Sci. Eng. A 384, 172 (2004). http://dx.doi.org/10.1016/j.msea.2004.06.011
Claude Carel, Mona Mouallem Bahout and Jean Gaude, Solid State Ionics 117, 47 (1999).
Wang Wenzhong, Zhan Yongjie and Wang Guanghou, Chem. Commun. 727 (2001). http://dx.doi.org/10.1039/B008215P
C. C. Vidyasagar, Y. Arthoba Naik, T. G. Venkatesh and R. Viswanath, Powder Tech. 214, 337 (2011). http://dx.doi.org/10.1016/j.powtec.2011.08.025
Tetsuya Kida, Takanori Oka and Masamitsu Nagano, J. Am. Ceram. Soc. 90, 107 (2007). http://dx.doi.org/10.1111/j.1551-2916.2006.01402.x
Wang Dong, Z. Q. Chen, D. D. Wang, J. Gong, C. Y. Cao and Z. Tang, et al., J. Magn. Magn. Mater. 332, 3642 (2010). http://dx.doi.org/10.1016/j.jmmm.2010.07.014
Masoud Sa lavati-Niasari, Fatemeh Davar and Mehdi Mazaheri, Mater. Lett. 62, 1890 (2008).
Siqingaowa, Zhaorigetu, H. Yao and Garidi, Front. Chem. China 3, 277 (2006). http://dx.doi.org/10.1007/s11458-006-0036-7
Fan Zhang and Junling Yang, Int. J. Chem. Kinet. 1, 18 (2009).
Z. C. Michael Hu, T. Michael Harris and H. Charles Byers, J. Colloid Interface Sci. 198, 87 (1998). http://dx.doi.org/10.1006/jcis.1997.5290
T. Prem Kumar, S. Saravanakumar and K. Sankaranarayanan, Appl. Surf. Sci. 257, 1923 (2011). http://dx.doi.org/10.1016/j.apsusc.2010.09.027
J. C. Fan and Z. Xie, Mater. Sci. Eng. B 150, 61 (2008). http://dx.doi.org/10.1016/j.mseb.2008.02.014
T. H. Mahato, Beer Singh, A. K. Srivastava, G. K. Prasad, A. R. Srivastava, K. Ganesan and R. Vijayaraghavan, J. Hazard. Mater. 192, 1890 (2011). http://dx.doi.org/10.1016/j.jhazmat.2011.06.078
Majid Ebrahimizadeh Abrishami, Seyed Mohammad Hosseini and Ahmad Kompany, J. App. Sci. 11, 1411 (2011). http://dx.doi.org/10.3923/jas.2011.1411.1415
Mehta, S. K. & Chaudhary, Savita. Sci. Topics Retrieved, January 20 (2012).
Chang-Woo Kwon, Tae-Sik Yoon, Sung-Soo Yim, Sang-Hyun Park and Ki-Bum Kim, J. Nanopart. Res. 11, 831 (2009). http://dx.doi.org/10.1007/s11051-008-9451-7
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vidyasagar, C.C., Arthoba Naik, Y., Venkatesha, T.G. et al. Solid-State Synthesis and Effect of Temperature on Optical Properties of CuO Nanoparticles. Nano-Micro Lett. 4, 73–77 (2012). https://doi.org/10.1007/BF03353695
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03353695