Abstract
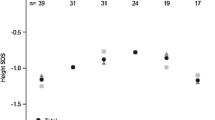
Aim: The optimal GH regimen, in terms of cost-effectiveness, in children with normal GH immunoreactivity but reduced bioactivity is still debated. Methods: In 12 GH-deficient (GHD) and 12 bioinactive GH children undergoing GH treatment we evaluated the increase in growth velocity, the difference between target height and final stature and the incremental cost-effectiveness ratio. Results: We found a significant (p<0.05) increase in growth velocity in both groups during the first year of GH treatment (non-GHD: from −1.7 to 5.4 SDS; GHD: from −1.46 to 4.74 SDS). There was no statistically significant variation between the two groups in the difference between final height and target height. We did not find any significant difference in cost/height gain between GHD (1925.28±653.15 euro) and bioinactive GH children (1639.55±631.44 euro). There were also no significant differences in cost/year of therapy between GHD (12347.68±2018.1 euro) and bioinactive GH children (11355.08±1747.61 euro). Conclusion: In children with reduced GH biological activity, confirmed by the increase of serum IGF-I levels during generation test, the cost of GH treatment is justified by the positive results obtained in growth and adult height as in classical GHD patients.
Similar content being viewed by others
References
Strasburger CJ, Bidlingmaier M. How robust are laboratory measures of growth hormone status? Horm Res 2005, 64(Suppl): 1–5.
Popii V, Baumann G: Laboratory measurement of growth hormone. Clin Chim Acta 2004, 350: 1–16.
Wood P. Growth hormone: its measurement and the need for assay harmonization. Ann Clin Biochem 2001, 38: 471–82.
Binder G, Benz MR, Elmlinger M, Pflaum CD, Strasburger CJ, Ranke MB. Reduced human growth hormone (hGH) bioactivity without a defect of the GH-1 gene in three patients with rhGH responsive growth failure. Clin Endocrinol (Oxf) 1999, 51: 89–95.
Kowarski AA, Schneider J, Ben-Galim E, Welden VV, Daughaday WH. Growth failure with normal serum RIA-GH and low somatomedin activity: somatomedin restoration and growth acceleration after exogenous GH. J Clin Endocrinol Metab 1978, 47: 461–4.
Lewis MD, Horan M, Millar DS, et al. A novel dysfunctional growth hormone variant (Ile179Met) exhibits a decreased ability to activate the extracellular signal-regulated kinase pathway. J Clin Endocrinol Metab 2004, 89: 1068–75.
Valenta LJ, Sigel MB, Lesniak MA, et al. Pituitary dwarfism in a patient with circulating abnormal growth hormone polymers. N Engl J Med 1985, 312: 214–7.
Hayek A, Peake GT, Greenberg RE. A new syndrome of short stature due to biologically inactive but immunoreactive growth hormone. Pediatr Res 1978, 12: 413–13.
Rudman D, Kutner MH, Blackston RD, Cushman RA, Bain RP, Patterson JH. Children with normal-variant short stature: treatment with human growth hormone for six months. N Engl J Med 1981, 305: 123–31.
Frazer T, Gavin JR, Daughaday WH, Hillman RE, Weldon W. Growth hormone-dependent growth failure. J Pediatr 1982, 101: 12–5.
Plotnick LP, Van Meter QL, Kowarski AA. Human growth hormone treatment of children with growth failure and normal growth hormone levels by immunoassay: lack of correlation with somatomedin generation. Pediatrics 1983, 71: 324–17.
Bright GM, Rogol AD, Johanson AJ, Blizzard RM. Short stature associated with normal growth hormone and decreased somatomedin-C concentrations: response to exogenous growth hormone. Pediatrics 1983, 71: 576–80.
Travaglino P, Buzi F, Meazza C, et al. Response to long-term growth hormone therapy in short children with reduced GH bioactivity. Horm Res 2006, 66: 189–94.
Albertsson-Wikland K, Aronson AS, Gustafsson J, et al. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab 2008, 93: 4342–50.
Juul A, Bang P, Hertel NT, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab 1994, 78: 744–52.
Juul A, Dalgaard P, Blum WF, et al. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab 1995, 80: 2534–42.
Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, and stages of puberty. Arch Dis Child 1976, 51: 170–9.
Schwarze CP, Wollmann HA, Binder G, Ranke MB. Short-term increments of insulin-like growth factor I (IGF-I) and IGF-binding pretein-3 predict the growth response to growth hormone (GH) therapy in GH-sensitive children. Acta Paediatr Suppl 1999, 88: 200–8.
Wit J-M. Growth Hormone Treatment of Idiopathic Short Stature in KIGS. In: Ranke MB, Wilton P eds. Growth hormone therapy in KIGS — 10 years’ experience. Heidelberg, Leipzig: Johann Ambrosius Barth Verlag. 1999, 225–43.
GH Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: Summary Statement of the GH Research Society. J Clin Endocrinol Metab 2000, 85: 3990–3.
Cotterill AM, Camacho-Ubner C, Dunquesnoy P, Savage MO. Changes in serum IGF-I and IGFBP-3 concentrations during the IGF-I generation test performed prospectively in children with short stature. Clin Endocrinol (Oxf) 1998, 48: 719–24.
Spiliotis BE, Alexandrides TK, Karystianos C, et al. The insulin-like growth factor-I (IGF-I) generation test as an indicator of growth hormone status. Hormones (Athens) 2009, 8: 117–28.
Tanaka T, Shiu RP, Gout PW, Beer CT, Noble RL, Friesen HG. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J Clin Endocrinol Metab 1980, 51: 1058–63.
Bozzola M, Zecca M, Locatelli F, et al. Evaluation of growth hormone bioactivity using the Nb2 cell bioassay in children with growth disorders. J Clin Endocrinol Metab 1998, 21: 765–70.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pagani, S., Meazza, C., Laarej, K. et al. Efficacy of long-term growth hormone therapy in short children with reduced growth hormone biological activity. J Endocrinol Invest 34, 366–369 (2011). https://doi.org/10.1007/BF03347461
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03347461