Abstract
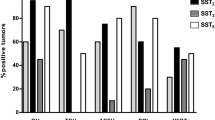
Introduction: About a third of acromegalic patients is resistant to available SS analogs (SA), octreotide (OCT) and lanreotide (LAN). Such resistance is related to reduction of SS receptor (SSTR) density or to a different expression of SSTR subtypes. There are 5 known SSTR subtypes. SSTR2 and SSTR5 are usually expressed in GH-secreting pituitary tumors, and both SA bind preferentially to SSTR2 and, to a lesser extent, to SSTR5. We herein describe an acromegalic patient who presented impressive tumor shrinkage without hormonal normalization during primary therapy with SA. Material and Methods: This 23-yr-old male acromegalic patient was treated with slow-release LAN (LAN-SR), 30 mg every 10 days for six months, followed by OCT-LAR, 30 mg every 28 days for an additional six months with a 75% tumor volume reduction but without GH and IGF-I normalization. Subsequently, he underwent pituitary surgery and expression of SSTR in the removed tumor was performed by real time RT-PCR by the 2-ΔCt method, using GAPDH as internal control. All PCR products were confirmed by automated sequencing. Results: SSTR expression revealed an unusual profile, with almost exclusively expression of SSTR3. Conclusions: These unusual clinical and receptor subtypes profile suggest an important role of SSTR3 on tumor shrinkage. The low affinity of LAN and OCT for this SSTR subtype could be compensated by its high expression in this GH-secreting pituitary macroadenoma.
Similar content being viewed by others
References
Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999, 20: 157–98.
Shimom I, Melmed S. Structure and function of somatostatina receptors in growth hormone control. J Endocrinol 1997, 155: S3–6.
Jallad RS, Musolino NC, Salgado LR, Bronstein MD. Treatment of acromegaly with octreotide-LAR: extensive experience in Brazilian institution. Clin Endocrinol (Oxf) 2005, 63: 168–75.
Danila DC, Haidar JNS, Zhang X, Katznelson L, Culler MD, Klibanski A. Somatostatin receptor-specific analogs: effects on cell proliferation and growth hormone secretion in human somatotroph tumors. J Clin Endocrinol Metab 2001, 86: 2976–81.
Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000, 85: 526–9.
Newman CB. Medical therapy for acromegaly. Endocrinol Metab Clin North Am 1999, 28: 171–90.
Essat S, Wilkins GE, Patel Y, Ur E, Rorstad O, Serri O. The diagnosis and management of acromegaly: a Canadian consensus report. Clin Invest Med 1996, 19: 259–70.
Sheppard MC, Stewart PM. Treatment options for acromegaly. Metabolism 1996, 45: 63–4.
Jaquet P, Saveanu A, Gunz G, et al. Human somatostatina receptor subtypes in acromegaly: distinct patterns of messenger ribonucleic acid expression and hormone suppressionidentify different tumoral phenotypes. J Clin Endocrinol Metab 2000, 85: 781–92.
Bevan JS. The antitumoral effects of somatostatina analogs therapy in acromegaly. J Clin Endocrinol Metab 2005, 90: 1856–63.
Melmed S, Jackson I, Kleinberg D, Klibanski A. Current treatment guidelines for acromegaly. J Clin Endocrinol Metab 1998, 8: 2646–52.
Antonov J, Goldstein DR, Oberli A, et al. Realible gene expression measurements from degraded RNA by quantitative real-time PCR depend on short amplicons and a proper normalization. Lab Invest 2005, 85: 1040–50.
Ponchel F, Toomes C, Bransfield K, et al. Real-time PCR based on SYBR-Green I fluorescence: An alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnology 2003, 1472–6750: 3–18.
Zheng H, Bailey A, Jiang MH, et al. Somatostatin receptor subtype 2 knockout mice are refractory to growth hormonenegative feedback on accurate neurons. Mol Endocrinol 1997, 11: 1709–17.
Zatelli MC, Piccin D, Tagliati F, et al. Somatostatin receptor subtype 1 selective activation in human growth hormone (GH) and prolactin (PRL) secreting pituitary adenomas: effects on cell viability, GH, and PRL secretion. J Clin Endocrinol Metab 2003, 88: 2797–802.
Ren SG, Taylor J, Dong J, Yu R, Culler MD, Melmed S. Functional association of somatostatin receptor subtype 2 and 5 in inhibiting human growth hormone secretion. J Clin Endocrinol Metab 2003, 88: 4239–45.
Stepanyan Z, Kocharyan A, Pyrski M, et al. Leptin-target neurons of rat hypothalamus express somatostatin receptors. J Neuroendocrinol 2003, 15: 1–9.
Sharma K, Patel YC, Srikant CB. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol Endocrinol 1996, 10: 1688–96.
Patel YC, Srikant CB. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hSSTR 1–5). Endocrinology 1994, 135: 2814–7.
Bruns C, Raulf F, Hoyer D, Schloos J, Lubbert H, Week-becker G. Binding properities of somatostatin receptor subtypes. Metabolism 1996, 45: 17–20.
Patel YC, Greenwood MT, Panetta R, Demehyshyn L, Niznik H, Srikant CB. The somatostatin receptor family. Life Sci 1995, 57: 1249–65.
Luciani P, Gelmini S, Ferrante E, et al. Expression of the antiapoptotic gene seladin-1 and octreotide-induced apoptosis in GH-secretinh and in non-functioning pituitary adenomas. J Clin Endocrinol Metab 2005, 10: 2005–633.
Teijeiro R, Rios R, Costoya JA, et al. Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem 2002, 12: 31–8.
Guillermet J, Saint-Laurent N, Rochaix P, et al. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. PROC Natl Acad Sci USA 2003, 100: 155–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casarini, A.P.M., Pinto, E.M., Jallad, R.S. et al. Dissociation between tumor shrinkage and hormonal response during somatostatin analog treatment in an acromegalic patient: Preferential expression of somatostatin receptor subtype 3. J Endocrinol Invest 29, 826–830 (2006). https://doi.org/10.1007/BF03347378
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03347378