Abstract
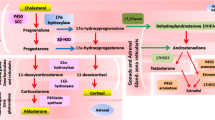
Objective: Increased peripheral metabolism of cortisol may explain compensatory ACTH-dependent adrenal steroidogenesis and hence hyperandrogenism in polycystic ovary syndrome (PCOS). Previous studies have described an increased 5α-reduction of cortisol or impaired regeneration of cortisol by 11β-HSD1 in PCOS. However, these observations may be confounded by obesity. Moreover, the relationship between alterations in cortisol metabolism and the extent of adrenal androgen hyper-secretion in response to ACTH has not been established. This study aimed to examine the association between cortisol metabolism and ACTH-dependent adrenal hyperandrogenism in PCOS, independently of obesity.Design: We compared 90 PCOS women (age 18–45 yr) stratified by adrenal androgen responses to ACTH1–24 and 45 controls matched for age and body weight. Methods: PCOS women were stratified as normal responders (NR), intermediate responders (IR), and high responders (HR) to 250 ug ACTH1–24: NR (no.=27) had androstenedione and DHEA responses within 2 SD of the mean in controls; IR (no.=43) had DHEA responses >2 SD above controls; HR (no.=20) had both androstenedione and DHEA responses >2 SD above controls. Results: All groups were similar for age, body weight, and body fat distribution. Basal testosterone, androstenedione, and 5α-dihydrotestosterone plasma levels were similarly elevated among the 3 groups of PCOS compared with controls, whereas basal DHEA-S was higher in HR (2.8±1.2 μg/ml) and IR (2.4±1.1 μg/ml) than in NR (1.8±0.8 μg/ml) and controls (1.7±0.6 μg/ml). The HR group had the lowest basal plasma cortisol levels (101±36 ng/ml vs IR 135±42 ng/ml, NR 144±48 ng/ml, and controls 165±48 ng/ml; all p<0.01), but the greatest cortisol response to ACTH1–24 (Δ(60–0)cortisol 173±60 ng/ml vs IR 136±51 ng/ml, NR 114±50 ng/ml, and controls 127±50 ng/ml; all p<0.01), and the highest urinary excretion of total and 5β-reduced cortisol metabolites (eg 5β-tetrahydrocortisol/cortisol ratio 25.2±15.3 vs IR 18.8±10.7, NR 19.7±11.4, and controls 17.2±13.7; all p<0.05). There were no differences in urinary excretion of 5α-reduced cortisol metabolites or in 5α-dihydrotestosterone/testosterone ratio between groups. Conclusions: Adrenal androgen excess in PCOS is associated with increased inactivation of cortisol by 5β-reductase that may lower cortisol blood levels and stimulate ACTH-dependent steroidogenesis.
Similar content being viewed by others
References
Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol 1992, 167: 1807–12.
Wild RA, Umstot ES, Andersen RN, Ranney GB, Givens JR. Androgen parameters and their correlation with body weight in one hundred thirty-eight women thought to have hyperandrogenism. Am J Obstet Gynecol 1983, 146: 602–5.
Hoffman DI, Klove K, Lobo RA. The prevalence and significance of elevated dehydroepiandrosterone sulfate levels in anovulatory women. Fertil Steril 1984, 42: 76–81.
Azziz R, Black V, Hines GA, Fox LM, Boots LR. Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab 1998, 83: 2317–23.
Walker BR. Activation of the hypothalamic-pituitary-adrenal axis: cause or consequence? Growth Horm IGF Res 2001, 11 (Suppl A): S91–5.
Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1 -a tissue-specific amplifier of glucocorticoid action. Endocrinology 2001, 142: 1371–6.
Russell DW, Wilson JD. Steroid 5α-reductase: two genes/two enzymes. Ann Rev Biochem 1994, 63: 25–61.
Charbonneau A, The VL Genomic organization of a human 5beta-reductase and its pseudogene and substrate selectivity of the expressed enzyme. Bioch Bioph Acta 2001, 1517: 228–35.
Rodin A, Thakkar H, Taylor N, Clayton R. Hyperandrogenism in polycystic ovary syndrome: evidence of dysregulation of 11β-hydroxysteroid dehydrogenase. N Engl J Med 1994, 330: 460–5.
Stewart PM, Shackleton CH, Beastall GH, Edwards CR. 5α-reductase activity in polycystic ovary syndrome. Lancet 1990, 335: 431–3.
Chin D, Shackleton C, Prasad VK, et al. Increased 5alpha-reductase and normal 11 beta-hydroxysteroid dehydrogenase metabolism of C19 and C21 steroids in a young population with polycystic ovarian syndrome. J Pediatr Endocrinol Metab 2000, 13: 253–9.
Tsilchorozidou T, Honour JW, Conway GS. Altered Cortisol metabolism in polycystic ovary syndrome: insulin enhances 5α-reduction but not the elevated adrenal steroid production rates. J Clin Endocrinol Metab 2003, 88: 5907–13.
Rask E, Olsson T, Söderberg S, et al. Tissue-specific dysregulation of Cortisol metabolism in human obesity. J Clin Endocrinol Metab 2001, 86: 1418–21.
Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone→cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab 1999, 84: 1022–7.
Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab 1998, 83: 1806–9.
Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med 1998, 338: 1876–80.
Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961, 21: 1440–7.
Gambineri A, Pelusi C, Genghini S, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004, 60: 241–9.
The Rotterdam ESHRE/ASMR-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004, 19: 41–7.
Pasquali R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab 2000, 85: 2767–74.
WHO Preventing and Managing the Global Epidemic. Report of a WHO consultation on obesity. WHO/NUT/NCD/98.1. 1997 World Health Organization, Geneva.
Gambineri A, Pelusi C, Manicardi E, et al. Glucose intolerance in a large cohort of mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes 2004, 53: 2353–8.
Pasquali R, Gambineri A, Anconetani B, et al. The natural history of the metabolic syndrome in young women with the polycystic ovary syndrome and the effect of long-term oestrogen-progestagen treatment. Clin Endocrinol (Oxf) 1999, 50: 517–27.
Vicennati V, Pasquali R. Abnormalities of the hypothalamic-pituitary-adrenal axis in nondepressed women with abdominal obesity and relations with insulin resistance: evidence for a central and peripheral alteration. J Clin Endocrinol Metab 2000, 85: 4093–8.
Boschi S, De Iasio R, Mesini P, et al. Measurement of steroid hormones in plasma by isocratic high performance liquid chromatography coupled to radioimmunoassay. Clin Chim Acta 1994, 231: 107–13.
Vermeulen A, Verdonck L, Kaufman JM. Acritical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999, 84: 3666–72.
Mather KJ, Hunt AE, Steinberg HO, et al. Repeatability characteristic of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab 2001, 86: 5457–64.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care 1999, 22: 1462–70.
Best R, Walker BR. Additional value of measurement of urinary cortisone and unconjugated cortisol metabolites in assessing the activity of 11β-hydroxysteroid dehydrogenase in vivo. Clin Endocrinol (Oxf) 1997, 47: 231–6.
Zumoff B, Fukushima DK, Hellman L. Intercomparison of four methods for measuring cortisol production. J Clin Endocrinol Metab 1974, 38: 169–75.
Ulick S, Tedde R, Wang JZ. Defective ring A reduction of cortisol as the major metabolic error in the syndrome of apparent mineralcorticoid excess. J Clin Endocrinol Metab 1992, 74: 593–9.
Palermo M, Shackleton CHL, Mantero F, Stewart PM. Urinary free cortisone and the assessment of 11β-hydroxysteroid dehydrogenase activity in man. Clin Endocrinol (Oxf) 1996, 45: 605–11.
Burger HG. Androgen production in women. Fertil Steril 2002, 77 (Suppl 4): S3–5.
Fassnacht M, Schlenz N, Schneider SB, Wudy SA, Allolio B, Arlt W. Beyond adrenal and ovarian androgen generation: increased peripheral 5α-reductase activity in women with polycystic ovary syndrome J Clin Endocrinol Metab 2003, 88: 2760–6.
Westerbacka J, Yki-Järvinen H, Vehkavaara S, et al. Body fat distribution and cortisol metabolism in healthy men: enhanced 5β-reductase and lower cortisol/cortisone metabolite ratios in men with fatty liver. J Clin Endocrinol Metab 2003, 88: 4924–31.
Livingstone DEW, Mclnnes KJ, Walker BR, Andrew R. Increased Aring reduction of glucocorticoids in obese Zucker rats: effects of insulin sensitization. Obes Res 2005, 13: 1523–6.
Stenberg A. On the modulating effects of ovaries on neonatal androgen programming of rat liver enzymes. Acta Endocrinol 1975, 78: 294–301.
Rosenfield RL. Ovarian and adrenal function in polycystic ovary syndrome. Endocrinol Metab Clin North Am 1999, 28: 265–93.
Moghetti P, Castello R, Negri C, et al. Insulin infusion amplifies 17α-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab 1996, 81: 881–6.
Nestler JE, McClanahan MA, Clore JN, Blackard WG. Insulin inhibits adrenal 17,20-lyase activity in man. J Clin Endocrinol Metab 1992, 74: 362–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gambineri, A., Forlani, G., Munarini, A. et al. Increased clearance of cortisol by 5β-reductase in a subgroup of women with adrenal hyperandrogenism in polycystic ovary syndrome. J Endocrinol Invest 32, 210–218 (2009). https://doi.org/10.1007/BF03346454
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03346454