Abstract
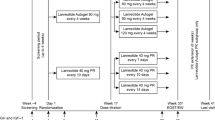
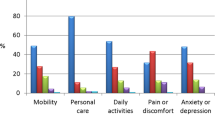
Objective: To evaluate efficacy and safety of lanreotide autogel (ATG) 120 mg injections every 4–8 weeks in somatostatin analogue-na"ive patients with acromegaly. Design: Open, non-comparative, phase III, multicenter clinical study. Methods: Fifty-one patients (28 women, aged 19–78 yr): 39 newly diagnosed (de novo) and 12 who had previously undergone unsuccessful surgery (post-op, 11 macro and 1 micro) were studied. ATG 120 mg was initially given every 8 weeks for 24 weeks and subsequently changed according to GH levels: if ≤2.5 μg/l every 8 weeks (group A, 17 patients); if 2.5–5 μg/l every 6 weeks (group B, 15 patients); and if >5 μg/l every 4 weeks (group C, 19 patients). Treatment duration was 48–52 weeks. The primary objective was to control GH and IGF-I levels (GH≤2.5 μg/l and IGF-I normalized for age/gender). Secondary objectives were to assess GH, IGF-I, and acid-labile subunit (ALS) decrease, improvement of clinical symptoms and quality of life (QoL). Results: GH levels normalized in 32 patients (63%), similarly in de novo and post-op patients (72% vs 50%, p=0.48); in 100% of group A, in 73% of group B and in 21 % of group C (p<0.0001). IGF-I levels normalized in 19 patients (37%), similarly in the de novo and post-op patients (33% vs 50%, p=0.48): in 65% of group A, 33% of group B, and in 16% of group C. Circulating GH levels decreased by 80±17%, IGF-I levels by 44±27%, and ALS by 30±17%. Symptoms (hyperhidrosis (68.6%), swelling (68.6%), asthenia (58.8%), spine arthralgia (54.9%), and paresthesias (52.9%) and QoL (from 9.1 ±7.9 to 6.1 ±6.6) significantly improved (p<0.001). No patient withdrew from the study because of adverse events (AE). The most frequent AE was diarrhea (76.2% of patients): at study end 16 mild and 1 moderate diarrhea were recorded. Gallstones developed in 12% of patients. Conclusion: ATG 120 mg in somatostatin-naïve patients with acromegaly controls GH secretion in 63% and IGF-I secretion in 37% during a 48–52 week period without any difference between de novo and post-op patients. The treatment was associated with improvement in clinical symptoms and QoL and with a good, safe profile.
Similar content being viewed by others
References
Melmed S, Casanueva FF, Cavagnini F, et al; Acromegaly Treatment Consensus Workshop Participants. Guidelines for Acromegaly Management. J Clin Endocrinol Metab 2002, 87: 4054–8.
Colao A, Lombardi G. Growth-hormone and prolactin excess. Lancet 1998, 352: 1455–61.
Freda PU. Somatostatin analogs in acromegaly. J Clin Endocrinol Metab 2002, 87: 3013–8.
Sheppard MC. Primary medical therapy for acromegaly. Clin Endocrinol (Oxf) 2003, 58: 387–99.
Heiman ML, Murphy WA, Coy DH. Differential binding of somatostatin agonists to somatostatin receptors in brain and in adenohypophysis. Neuroendocrinology 1987, 45: 429–36.
Antonijoan RM, Barbanoj MJ, Cordero JA, et al. Pharmacokinetics of a new Autogel formulation of the somatostatin analogue lanreotide after a single subcutaneous dose in healthy volunteers. J Pharm Pharmacol 2004, 56: 471–6.
Caron P, Beckers A, Cullen DR, et al. Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in the management of acromegaly. J Clin Endocrinol Metab 2002, 87: 99–104.
Lucas T, Astorga R; Spanish-Portuguese Multicentre Autogel Study Group on Acromegaly. Efficacy of lanreotide Autogel administered every 4–8 weeks in patients with acromegaly previously responsive to lanreotide microparticles 30 mg: a phase III trial. Clin Endocrinol (Oxf) 2006, 65: 320–6.
Caron P, Cogne M, Raingeard I, Bex-Bachellerie V, Kuhn JM. Effectiveness and tolerability of 3-year lanreotide Autogel treatment in patients with acromegaly. Clin Endocrinol (Oxf) 2006, 64: 209–14.
Ashwell SG, Bevan JS, Edwards OM, et al. The efficacy and safety of lanreotide Autogel in patients with acromegaly previously treated with octreotide LAR. Eur J Endocrinol 2004, 150: 473–80.
Alexopoulou O, Abrams P, Verhelst J, et al. Efficacy and tolerability of lanreotide Autogel therapy in acromegalic patients previously treated with octreotide LAR. EurJ Endocrinol 2004, 151: 317–24.
Van Thiel SW, Romijn JA, Biermasz NR, et al. Octreotide long-acting repeatable and lanreotide Autogel are equally effective in controlling growth hormone secretion in acromegalic patients. Eur J Endocrinol 2004, 150: 489–95.
Ciccarelli A, Daly A, Beckers A. Lanreotide Autogel for Acromegaly. A new addition to the treatment armamentarium. Treat Endocrinol 2004, 3: 77–81.
Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000, 85: 526–9.
Barreca A, Ponzani P, Arvigo M, Giordano G, Minuto F. Effect of the acid-labile subunit on the binding of insulin-like growth factor (IGF)-binding protein-3 to [125l]IGF-I. J Clin Endocrinol Metab 1995, 80: 1318–24.
Newman CB, Melmed S, George A, et al. Octreotide as primary therapy for acromegaly. J Clin Endocrinol Metab 1998, 83: 3034–40.
Colao A, Ferone D, Marzullo P, et al. Long-term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab 2001, 86: 2779–86.
Baldelli R, Colao A, Razzore P, et al. Two-year follow-up of acromegalic patients treated with slow release lanreotide (30 mg). J Clin Endocrinol Metab 2000, 85: 4099–103.
Ayuk J, Stewart SE, Stewart PM, Sheppard MC European Sandostatin LAR Group. Efficacy of Sandostatin LAR (long-acting somatostatin analogue) is similar in patients with untreated acromegaly and in those previously treated with surgery and/or radiotherapy. Clin Endocrinol (Oxf) 2004, 60: 375–81.
Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, Rabinowitz D. Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab 2005, 90: 4465–73.
Cozzi R, Montini M, Attanasio R, et al. Primary treatment of acromegaly with octreotide LAR: a long-term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkage. J Clin Endocrinol Metab 2006, 91: 1397–403.
Murray RD, Melmed S. A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab 2008, 93: 2957–68.
Arosio M, Garrone S, Bruzzi P, Faglia G, Barreca A. Diagnostic value of the acid-labile subunit in acromegaly: evaluation in comparison with insulin-like growth factor (IGF) I, and IGF-binding protein-1, -2, and -3. JClin Endocrinol Metab 2001, 86: 1091–8.
Epaminonda P, Porretti S, Cappiello V, Beck-Peccoz P, Faglia G, Arosio M. Efficacy of radiotherapy in normalizing serum IGF-I, acidlabile subunit (ALS) and IGFBP-3 levels in acromegaly. Clin Endocrinol (Oxf) 2001, 55: 183–9.
Feelders RA, Bidlingmaier M, Strasburger CJ, et al. Postoperative evaluation of patients with acromegaly: clinical significance and timing of oral glucose tolerance testing and measurement of (free) insulin-like growth factor I, acid-labile subunit, and growth hormone-binding protein levels. J Clin Endocrinol Metab 2005, 90: 6480–9.
Ronchi CL, Boschetti M, Degli Uberti EC, et al.; Italian Multicenter Autogel Study Group In Acromegaly. Efficacy of a slow-release formulation of lanreotide (autogel® 1 20 mg) in patients with acromegaly previously treated with octreotide LAR: an open, multicenter longitudinal study. Clin Endocrinol (Oxf) 2007, 67: 512–9.
Webb SM, Badia X, Surinach NL; Spanish AcroQol Study Group. Validity and clinical applicability of the acromegaly quality of life questionnaire, AcroQoL: a 6-month prospective study. Eur J Endocrinol 2006, 155: 269–77.
Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 2004, 25: 102–52.
Colao A, Auriemma RS, Rebora A, et al. Significant tumour shrinkage after 12 months of Lanreotide Autogel-120 mg treatment given first-line in acromegaly. Clin Endocrinol (Oxf) 2008 Dec 15. [Epub ahead of print], doi: 10.1111/j.1365-2265.2008.03503.x
Colao A, Martino E, Cappabianca P, Cozzi R, Scanarini M, Ghigo E; A.L.I.C.E. Study Group. First-line therapy of acromegaly: a statement of the A.L.I.C.E. (Acromegaly primary medical treatment Learning and Improvement with Continuous Medical Education) Study Group. J Endocrinol Invest 2006, 29: 1017–20.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study (A-93-52030-077) has been registered in www.clinicaltrials.gov as NCT00499993
Rights and permissions
About this article
Cite this article
Lombardi, G., Minuto, F., Tamburrano, G. et al. Efficacy of the new long-acting formulation of lanreotide (Lanreotide Autogel) in somatostatin analogue-naive patients with acromegaly. J Endocrinol Invest 32, 202–209 (2009). https://doi.org/10.1007/BF03346453
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03346453