Abstract
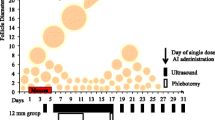
Dienogest is a selective progestin that has been shown to arrest ovarian follicular development in women, without affecting gonadotropin secretion. As luteal progesterone or exogeneous progestins are known to suppress ovarian folliculogenesis via the inhibition of gonadotropin secretion, this action of dienogest on ovaries seems to be unique. To examine the underlying mechanism of the antifolliculogenic effect of dienogest, female cynomolgus monkeys were treated with a single oral dose of 0.1 mg/kg dienogest on day 7 of the menstrual cycle. Plasma FSH, estradiol (E2), and progesterone levels were measured up to 15 days after dosing. In an additional experiment, ovaries were excised 24 h after dosing for histological examinations. As a result, plasma E2 level declined within 24 h after dosing, while dienogest did not decreased FSH level prior to E2 decline. After decline of E2 level, the low level of E2 was sustained for more than 11 days. It is considered that a single oral dose of dienogest induced atresia of the dominant follicle. In the histological examination, two out of three animals showed decline in E2 level. The ovarian dominant follicles from these animals showed apoptotic changes in granulosa cells with scattered aromatase expression within 24 h after dosing. These results indicate that the induction of atresia of the ovarian dominant follicle by direct action would be a possible mechanism of dienogest to inhibit plasma E2 level.
Similar content being viewed by others
References
Sitruk-Ware R. Pharmacological profile of progestins. Maturitas 2004, 47: 277–83.
Oettel M, Kurischko A. STS 557, a new orally active progestin with antiprogestational and contragestational properties in rabbits. Contraception 1980, 21: 61–9.
Gupta G, Srivastava A, Setty BS. Effect of antiandrogens on some key enzymes of glycolysis in epididymis and ventral prostate of rat. Indian J Exp Biol 1989, 27: 324–8.
Katsuki Y, Sasagawa S, Takano Y, et al. Animal studies on the endocrinological profile of dienogest, a novel synthetic steroid. Drugs Exp Clin Res 1997, 23: 45–62.
Oettel M, Carol W, Elger W, et al. A 19-norprogestin without 17α-ethinyl group II: Dienogest from a pharmacodynamic point of view. Drugs of Today 1995, 31: 517–36.
Wilks JW, Spilman CH, Campbell JA. Arrest of folliculogenesis and inhibition of ovulation in the monkey following weekly administration of progestins. Fertil Steril 1983, 40: 688–92.
Suzuki T, Sasano H, Tamura M, et al. Temporal and spatial localization of steroidogenic enzymes in premenopausal human ovaries: in situ hybridization and immunohistochemical study. Mol Cell Endocrinol 1993, 97: 135–43.
Petta CA, Hays M, Brache V, et al. Delayed first injection of the once-a-month injectable contraceptive containing 25 mg of medroxyprogesterone acetate and 5 mg of E(2)-cypionate: effects on ovarian function. Fertil Steril 2001, 75: 744–8.
Baerwald AR, Olatunbosun OA, Pierson RA. Effects of oral contraceptives administered at defined stages of ovarian follicular development. Fertil Steril 2006, 86: 27–35.
di Zerega GS, Hodgen GD. Fluorescence localization of luteinizing hormone/human chorionic gonadotropin uptake in the primate ovary. II. Changing distribution during selection of the dominant follicle. J Clin Endocrinol Metab 1980, 51: 903–7.
Goodman AL, Descalzi CD, Johnson DK, Hodgen GD. Composite pattern of circulating LH, FSH, estradiol, and progesterone during the menstrual cycle in cynomolgus monkeys. Proc Soc Exp Biol Med 1977, 155: 479–81.
Goodman AL, Hodgen GD. Systemic versus intraovarian progesterone replacement after luteectomy in rhesus monkeys: differential patterns of gonadotropins and follicle growth. J Clin Endocrinol Metab 1977, 45: 837–40.
Goodman AL, Nixon WE, Hodgen GD. Between-ovary interaction in the regulation of follicle growth, corpus luteum function, and gonadotropin secretion in the primate ovarian cycle. III. Temporal and spatial dissociation of folliculogenesis and negative feedback regulation of tonic gonadotropin release after luteectomy in rhesus monkeys. Endocrinology 1979, 105: 69–73.
Goodman AL, Hodgen GD. Antifolliculogenic action of progesterone despite hypersecretion of FSH in monkeys. Am J Physiol 1982, 243: E387–97.
Zeleznik AJ, Resko JA. Progesterone does not inhibit gonadotropin-induced follicular maturation in the female rhesus monkey (Macaca mulatta). Endocrinology 1980, 106: 1820–6.
Filicori M, Bolelli G, Franceschetti F, Lafisca S. The ultradian pulsatile release of gonadotropins in normal female subjects. Acta Eur Fertil 1979, 10: 29–33.
Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab 1984, 58: 378–83.
Pohl CR, Richardson DW, Hutchison JS, Germak JA, Knobil E. Hypophysiotropic signal frequency and the functioning of the pituitary-ovarian system in the rhesus monkey. Endocrinology 1983, 112: 2076–80.
Spies HG, Niswender GD. Blockade of the surge of preovulatory serum luteinizing hormone and ovulation with exogenous progesterone in cycling rhesus (Macaca mulatta) monkeys. J Clin Endocrinol Metab 1971, 32: 309–16.
Clark JR, Dierschke DJ, Wolf RC. Hormonal regulation of ovarian folliculogenesis in rhesus monkeys: III. Atresia of the preovulatory follicle induced by exogenous steroids and subsequent follicular development. Biol Reprod 1981, 25: 332–41.
Resko JA, Ellinwood WE, Knobil E. Differential effects of progesterone on secretion of gonadotropic hormones in the rhesus monkey. Am J Physiol 1981, 240: E489–92.
Oettel M, Carol W, Graser T, et al. [Effect of ethinyl estradioldienogest combination on serum androgen concentrations]. Zentralbl Gynakol 1997, 119: 597–606.
Parrott JA, Vigne JL, Chu BZ, Skinner MK. Mesenchymal-epithelial interactions in the ovarian follicle involve keratinocyte and hepatocyte growth factor production by thecal cells and their action on granulosa cells. Endocrinology 1994, 135: 569–75.
Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006, 132: 191–206.
Armstrong DG, Webb R. Ovarian follicular dominance: the role of intraovarian growth factors and novel proteins. Rev Reprod 1997, 2: 139–46.
Sudo N, Shimizu T, Kawashima C, Kaneko E, Tetsuka M, Miyamoto A. Insulin-like growth factor-I (IGF-I) system during follicle development in the bovine ovary: relationship among IGF-I, type 1 IGF receptor (IGFR-1) and pregnancy-associated plasma protein-A (PAPP-A). Mol Cell Endocrinol 2007, 264: 197–203.
Buratini J Jr, Pinto MG, Castilho AC, et al. Expression and function of fibroblast growth factor 10 and its receptor, fibroblast growth factor receptor 2B, in bovine follicles. Biol Reprod 2007, 77: 743–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasagawa, S., Shimizu, Y., Nagaoka, T. et al. Dienogest, a selective progestin, reduces plasma estradiol level through induction of apoptosis of granulosa cells in the ovarian dominant follicle without follicle-stimulating hormone suppression in monkeys. J Endocrinol Invest 31, 636–641 (2008). https://doi.org/10.1007/BF03345616
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345616