Abstract
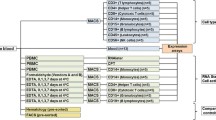
GH replacement therapy exhibits a wide spectrum of response in terms of growth. Nevertheless, standardized doses are still given in clinical practice. In order to optimize the therapy, it is necessary to identify its markers of responsiveness. Given the presence of GH receptors in the circulating lymphocytes, accessible by means of a simple blood withdrawal, blood becomes the tissue of choice as a source of RNA for in vivo gene expression analysis. Hence, the purpose of the present paper is to develop a method of preparation of RNA from lymphocytes suitable for microarray analysis, focusing on the reduction of the blood volume withdrawal in order to perform the analysis on pediatric subjects. After lymphocyte isolation and total RNA extraction from 6 ml of blood, we carried out an amplification procedure preserving the relative abundance of each transcript. Thereafter, we hybridized the labeled amplified RNA on an oligo chip (Human 30K A, MWG-Biotech), but the unsuccessful detection of a good signal to noise ratio indicates that labeled RNA is still insufficient. Therefore, we suggest performing pools of total RNA from different subjects with similar responsiveness to the therapy. It can be speculated that, upon comparison of the obtained data with those derived from pools of controls properly responding to the therapy, specific hallmarks of the condition of low responsiveness, devoid of inter-individual variability, will be evidenced.
Similar content being viewed by others
References
Casanueva FF. Physiology of growth hormone secretion and action. Endocrinol Metab Clin North Am 1992, 21: 483–517.
Davidson MB. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr Rev 1987, 8: 115–31.
Kelly PA, Djiane J, Postel-Vinay MC, Edery M. The prolactin/growth hormone receptor family. Endocr Rev 1991, 12: 235–51.
Nyberg F, Burman P. Growth hormone and its receptors in the central nervous system: Location and functional significance. Horm Res 1996, 45: 18–22.
Ohlsson C, Lövstedt K, Holmes PV, Nilsson A, Carlsson L, Törnell J. Embryonic stem cells express growth hormone receptors: Regulation by retinoic acid. Endocrinology 1993, 133: 2897–903.
Ochoa R, Zárate A, Cerbón MA, et al. Expression of growth hormone receptor isoform exon-3-excluding and exon-3-retaining messenger RNAs in peripheral lymphocytes from normal and acromegalic subjects. Horm Res 2003, 60: 68–72.
Ban E, Gagnerault MC, Jammes H, Postel-Vinay MC, Haour F, Dardenne M. Specific binding sites for growth hormone in cultured mouse thymic epithelial cells. Life Sci 1991, 48: 2141–8.
Rapaport R, Sills IN, Green L, et al. Detection of human growth hormone receptors on IM-9 cells and peripheral blood mononuclear cell subsets by flow cytometry: Correlation with growth hormone-binding protein levels. J Clin Endocrinol Metab 1995, 80: 2612–9.
Chen HT, Schuler LA, Schultz RD. Growth hormone receptor and regulation of gene expression in fetal lymphoid cells. Mol Cell Endocrinol 1998, 137: 21–9.
Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995, 270: 467–70.
Flores-Morales A, Ståhlberg N, Tollet-Egnell P, et al. Microarray analysis of the in vivo effects of hypophysectomy and growth hormone treatment on gene expression in the rat. Endocrinology 2001, 142: 3163–76.
Tollet-Egnell P, Flores-Morales A, Ståhlberg N, Malek RL, Lee N, Norstedt G. Gene expression profile of the aging process in rat liver: normalizing effects of growth hormone replacement. Mol Endocrinol 2001, 15: 308–18.
Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol Endocrinol 2004, 18: 747–60.
Tollet-Egnell P, Parini P, Ståhlberg N, et al. Growth hormone-mediated alteration of fuel metabolism in the aged rat as determined from transcript profiles. Physiol Genomics 2004, 16: 261–7.
Tivesten Å, Barlind A, Caidahl K, et al. Growth hormone-induced blood pressure decrease is associated with increased mRNA levels of the vascular smooth muscle KATP channel. J Endocrinol 2004, 183: 195–202.
Huo JS, McEachin RC, Cui TX, et al. Profiles of growth hormone (GH)-regulated genes reveal time-dependent responses and identify a mechanism for regulation of activating transcription factor 3 by GH. J Biol Chem 2006, 281: 4132–41.
Yoshioka S, Takahashi Y, Okimura Y, et al. Gene expression profile in the heart of spontaneous dwarf rat: in vivo effects of growth hormone. Biochem Biophys Res Commun 2006, 341: 88–93.
Govoni KE, Lee SK, Chadwick RB, et al. Whole genome microarray analysis of growth hormone-induced gene expression in bone: T-box3, a novel transcription factor, regulates osteoblast proliferation. Am J Physiol Endocrinol Metab 2006, 291: E128–36.
Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 2006, 20: 1333–51.
Ellestad LE, Carre W, Muchow M, et al. Gene expression profiling during cellular differentiation in the embryonic pituitary gland using cDNA microarrays. Physiol Genomics 2006, 25: 414–25.
Giorgi RR, Correa-Giannella ML, Casarini AP, et al. Metallothionein isoform 3 gene is differentially expressed in corticotropin-producing pituitary adenomas. Neuroendocrinology 2005, 82: 208–14.
Bjarnason R, Andersson B, Kim HS, et al. Cartilage oligomeric matrix protein increases in serum after the start of growth hormone treatment in prepubertal children. J Clin Endocrinol Metab 2004, 89: 5156–60.
Kriström B, Jansson C, Rosberg S, Albertsson-Wikland K. Growth response to growth hormone (GH) treatment relates to serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in short children with various GH secretion capacities. Swedish Study Group for Growth Hormone Treatment. J Clin Endocrinol Metab 1997, 82: 2889–98.
Wikland K, Kriström B, Rosberg S, Svensson B, Nierop AF. Validated multivariate models predicting the growth response to GH treatment in individual short children with a broad range in GH secretion capabilities. Pediatr Res 2000, 48: 475–84.
Ranke MB, Lindberg A, Chatelain P, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab 1999, 84: 1174–83.
Wilfinger WW, Mackey K, Chomczynski P. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques 1997, 22: 474–81.
Baugh LR, Hill AA, Brown EL, Hunter CP. Quantitative analysis of mRNA amplification by in vitro transcription. Nucl Acids Res 2001, 29: E29.
Pabón C, Modrusan Z, Ruvolo MV, et al. Optimized T7 amplification system for microarray analysis. Biotechniques 2001, 31: 874–9.
Poirier GM, Erlander MG. Postdifferential display: parallel processing of candidates using small amounts of RNA. Methods 1998, 16: 444–52.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camilot, M., Teofoli, F., Longobardi, S. et al. A technique of mRNA extraction and labeling from circulating lymphocytes of children treated with growth hormone replacement therapy for microarray analysis. J Endocrinol Invest 31, 1–7 (2008). https://doi.org/10.1007/BF03345559
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345559