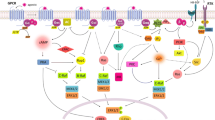
Abstract
Regulation of hormone secretion is a complex process that comprises the sequential participation of numerous subcellular mechanisms. Hormone secretion is dictated by extracellular stimuli that are transduced intracellularly into activation/deactivation of different mechanisms, such as hormone expression, processing and exocytosis, which will ultimately determine the precise availability of hormone to be secreted. Malfunction in any of these steps may result in deficient or excessive hormone release and the subsequent appearance of endocrine disorders. Given the complexity of this system, it is difficult to find appropriate cellular models wherein to investigate the multiple components of the secretory process in a physiologically relevant, experimentally manipulable setting. In this review, we present recent evidence on the use of the intermediate lobe (IL) of the pituitary as a powerful tool to understand different aspects of the regulated secretory pathway. IL is composed of a single endocrine cell type, α-melanocyte stimulating hormone (α-MSH)-producing melanotropes, a fact that greatly facilitates its study. Furthermore, melanotropes can be separated using classic cell separation techniques into two cell subtypes showing opposite morphophysiological phenotypes of hypo- and hypersecretory cells. Comparison of their gene expression fingerprints has unveiled the existence of certain genes preferentially expressed in each melanotrope subtype. Because of their direct participation in the secretory pathway, we postulate that characterization of these gene products in an endocrine cell type may represent novel and useful markers for reliably determining the general secretory status in an endocrine gland, as well as a valuable new tool to further investigate this complex process.
Similar content being viewed by others
References
Castro-Fernandez C, Maya-Nunez G, Conn PM. Beyond the signal sequence: protein routing in health and disease. Endocr Rev 2005, 26: 479–503.
Taylor SI. Lilly Lecture: molecular mechanisms of insulin resistance. Lessons from patients with mutations in the insulin-receptor gene. Diabetes 1992, 41: 1473–90.
Duquesnoy P, Sobrier ML, Amselem S, et al. Defective membrane expression of human growth hormone (GH) receptor causes Laron-type GH insensitivity syndrome. Proc Natl Acad Sci U S A 1991, 88: 10272–6.
Cheng SY. Thyroid hormone receptor mutations and disease: beyond thyroid hormone resistance. Trends Endocrinol Metab 2005, 16: 176–82.
Mbikay M, Seidah NG, Chretien M. Neuroendocrine secretory protein 7B2: structure, expression and functions. Biochem J 2001, 357: 329–42.
Semiz S, Park JG, Nicoloro SM, et al. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J 2003, 22: 2387–99.
Seog DH, Lee DH, Lee SK. Molecular motor proteins of the kinesin superfamily proteins (KIFs): structure, cargo and disease. J Korean Med Sci 2004, 19: 1–7.
Wilson JF, Morgan MA. α-Melanotropin-like substances in the pituitary and plasma of Xenopus laevis in relation to colour change responses. Gen Comp Endocrinol 1979, 38: 172–82.
van Zoest ID, Heijmen PS, Cruijsen PM, et al. Dynamics of background adaptation in Xenopus laevis: role of catecholamines and melanophore-stimulating hormone. Gen Comp Endocrinol 1989, 76: 19–28.
Saland LC. The mammalian pituitary intermediate lobe: an update on innervation and regulation. Brain Res Bull 2001, 54: 587–93.
Seidah NG, Benjannet S, Hamelin J, et al. The subtilisin/kexin family of precursor convertases. Emphasis on PC1, PC2/7B2, POMC and the novel enzyme SKI-1. Ann N Y Acad Sci 1999, 885: 57–74.
Dores RM, Harris S. Differential N-acetylation of α-MSH and β-endorphin in the intermediate pituitary of the turtle. Pseudemys scripta. Peptides 1993, 14: 849–55.
Glembotski CC. Acetylation of α-melanotropin and β-endorphin in the rat intermediate pituitary. Subcellular localization. J Biol Chem 1982, 257: 10493–500.
Martens GJ, Jenks BG, van Overbeeke AP. N α-acetylation is linked to α-MSH release from pars intermedia of the amphibian pituitary gland. Nature 1981, 294: 558–60.
Vaudry H, Jenks BG, van Overbeeke AP. The frog pars intermedia contains only the non-acetylated form of α-MSH: acetylation to generate α-MSH occurs during the release process. Life Sci 1983, 33 (Suppl 1): 97–100.
Dores RM, Truong T, Steveson TC. Detection and partial characterization of proopiomelanocortin-related end-products from the pars intermedia of the toad, Bombina orientalis. Gen Comp Endocrinol 1992, 87: 197–207.
Doerr-Schott J. Immunohistochemistry of the adenohypophysis of non-mammalian vertebrates. Acta Histochem 1980, 22 (Suppl): 185–223.
Vazquez-Martinez R, Peinado JR, Gonzalez De Aguilar JL, et al. Melanotrope cell plasticity: a key mechanism for the physiological adaptation to background color changes. Endocrinology 2001, 142 3060–7.
Moriarty GC. Immunocytochemistry of the pituitary glycoprotein hormones. J Histochem Cytochem 1976, 24: 846–63.
Hopkins CR, Farquhar MG. Hormone secretion by cells dissociated from rat anterior pituitaries. J Cell Biol 1973, 59: 277–303.
Snyder G, Hymer WC, Snyder J. Functional heterogeneity in somatotrophs isolated from the rat anterior pituitary. Endocrinology 1977, 101: 788–99.
Neill JD, Frawley LS. Detection of hormone release from individual cells in mixed populations using a reverse hemolytic plaque assay. Endocrinology 1983, 112: 1135–7.
Holl RW, Thorner MO, Mandell GL, et al. Spontaneous oscillations of intracellular calcium and growth hormone secretion. J Biol Chem 1988, 263: 9682–5.
Castano JP, Kineman RD, Frawley LS. Dynamic monitoring and quantification of gene expression in single, living cells: a molecular basis for secretory cell heterogeneity. Mol Endocrinol 1996, 10: 599–605.
Schwartz J, Gracia-Navarro F. Ain’t misbehavin’: reflections on the functional differences among anterior pituitary cells. Mol Cell Endocrinol 1996, 123: 1–6.
Chronwall BM, Hook GR, Millington WR. Dopaminergic regulation of the biosynthetic activity of individual melanotropes in the rat pituitary intermediate lobe: a morphometric analysis by light and electron microscopy and in situ hybridization. Endocrinology 1988, 123: 1992–2002.
Chronwall BM, Millington WR, Griffin WS, et al. Histological evaluation of the dopaminergic regulation of proopiomelanocortin gene expression in the intermediate lobe of the rat pituitary, involving in situ hybridization and [3H]thymidine uptake measurement. Endocrinology 1987, 120: 1201–11.
de Rijk E P, Jenks BG, Wendelaar Bonga SE. Morphology of the pars intermedia and the melanophore-stimulating cells in Xenopus laevis in relation to background adaptation. Gen Comp Endocrinol 1990, 79: 74–82.
Gonzalez de Aguilar JL, Tonon MC, Ruiz-Navarro A, et al. Morphological and functional heterogeneity of frog melanotrope cells. Neuroendocrinology 1994, 59: 176–82.
Gonzalez de Aguilar JL, Malagon MM, Vázquez-Martínez RM, et al. Two frog melanotrope cell subpopulations exhibiting distinct biochemical and physiological patterns in basal conditions and under thyrotropin-releasing hormone stimulation. Endocrinology 1997, 138: 970–7.
Gonzalez de Aguilar JL, Malagon MM, Vázquez-Martínez RM, et al. Differential effects of dopamine on two frog melanotrope cell subpopulations. Endocrinology 1999, 140: 159–64.
Vázquez-Martínez R, Malagon MM, Castaño JP, et al. Amphibian melanotrope subpopulations respond differentially to hypothalamic secreto-inhibitors. Neuroendocrinology 2001, 73: 426–34.
Iturriza FC. Electron-microscopic study of the pars intermedia of the pituitary of the toad Bufo arenarum. Gen Comp Endocrinol 1964, 47: 492–502.
Tonon MC, Bosler O, Stoeckel ME, et al. Co-localization of tyrosine hydroxylase, GABA and neuropeptide Y within axon terminals innervating the intermediate lobe of the frog Rana ridibunda, J Comp Neurol 1992, 319: 599–605.
de Rijk E P, van Strien FJ, Roubos EW. Demonstration of coexisting catecholamine (dopamine), amino acid (GABA), and peptide (NPY) involved in inhibition of melanotrope cell activity in Xenopus laevis: a quantitative ultrastructural, freeze-substitution immunocytochemical study. J Neurosci 1992, 12: 864–71.
Jenks B, Buzzi M, Dotman C, et al. The significance of multiple inhibitory mechanisms converging on the melanotrope cell of Xenopus laevis. Ann N Y Acad Sci USA 1998, 839: 229–34.
Tonon MC, Desrues L, Lamacz M, et al. Multihormonal regulation of pituitary melanotrophs. Ann N Y Acad Sci USA 1993, 680: 175–87.
Vázquez-Martínez R, Castaño JP, Tonon MC, et al. Melanotrope secretory cycle is regulated by physiological inputs via the hypothalamus. Am J Physiol Endocrinol Metab 2003, 285: E1039–46.
Solomon S. POMC-derived peptides and their biological action. Ann N Y Acad Sci 1999, 885: 22–40.
Vázquez-Martínez RM, Malagon MM, van Strien FJ, et al. Analysis by mass spectrometry of POMC-derived peptides in amphibian melanotrope subpopulations. Life Sci 1999, 64: 923–30.
Steiner DF, Rouille Y, Gong Q, et al. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes Metab 1996, 22: 94–104.
Westphal CH, Muller L, Zhou A, et al. The neuroendocrine protein 7B2 is required for peptide hormone processing in vivo and provides a novel mechanism for pituitary Cushing’s disease. Cell 1999, 96: 689–700.
Mbikay M, Sirois F, Yao J, et al. Comparative analysis of expression of the proprotein convertases furin, PACE4, PC1 and PC2 in human lung tumours. Br J Cancer 1997, 75: 1509–14.
Cheng M, Watson PH, Paterson JA, et al. Pro-protein convertase gene expression in human breast cancer. Int J Cancer 1997, 71: 966–71.
Bassi DE, Mahloogi H, Al-Saleem L, et al. Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol Carcinog 2001, 31: 224–32.
Khatib AM, Siegfried G, Chretien M, et al. Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am J Pathol 2002, 160: 1921–35.
Takumi I, Steiner DF, Sanno N, et al. Localization of prohormone convertases 1/3 and 2 in the human pituitary gland and pituitary adenomas: analysis by immunohistochemistry, immunoelectron microscopy, and laser scanning microscopy. Mod Pathol 1998, 11: 232–8.
Jin L, Kulig E, Qian X, et al. Distribution and regulation of proconvertases PC1 and PC2 in human pituitary adenomas. Pituitary 1999, 1: 187–95.
Seidah NG, Chretien M, Day R. The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie 1994, 76: 197–209.
Schafer MK, Day R, Cullinan WE, et al. Gene expression of prohormone and proprotein convertases in the rat CNS: a comparative in situ hybridization analysis. J Neurosci 1993, 13: 1258–79.
Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience 1992, 49: 497–528.
Iacangelo AL, Eiden LE. Chromogranin A: current status as a precursor for bioactive peptides and a granulogenic/sorting factor in the regulated secretory pathway. Regul Pept 1995, 58: 65–88.
Fischer-Colbrie R, Laslop A, Kirchmair R. Secretogranin II: molecular properties, regulation of biosynthesis and processing to the neuropeptide secretoneurin. Prog Neurobiol 1995, 46: 49–70.
Rosa P, Gerdes HH. The granin protein family: markers for neuroendocrine cells and tools for the diagnosis of neuroendocrine tumors. J Endocrinol Invest 1994, 17: 207–25.
Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev 1991, 12: 181–7.
Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med 2003, 348: 1134–49.
Loh YP, Kim T, Rodriguez YM, et al. Secretory granule biogenesis and neuropeptide sorting to the regulated secretory pathway in neuroendocrine cells. J Mol Neurosci 2004, 22: 63–71.
Huh YH, Jeon SH, Yoo SH. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem 2003, 278: 40581–9.
Beuret N, Stettler H, Renold A, et al. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem 2004, 279: 20242–9.
Nicol L, McNeilly JR, Stridsberg M, et al. Influence of steroids and GnRH on biosynthesis and secretion of secre-togranin II and chromogranin A in relation to LH release in LβT2 gonadotroph cells. J Endocrinol 2002, 174: 473–83.
Crawford JL, McNeilly JR, Nicol L, et al. Promotion of intragranular co-aggregation with LH by enhancement of secretogranin II storage resulted in increased intracellular granule storage in gonadotrophs of GnRH-deprived male mice. Reproduction 2002, 124: 267–77.
Day R, Gorr SU. Secretory granule biogenesis and chromogranin A: master gene, on/off switch or assembly factor? Trends Endocrinol Metab 2003, 14: 10–3.
Kim T, Tao-Cheng JH, Eiden LE, et al. The role of chromogranin A and the control of secretory granule genesis and maturation. Trends Endocrinol Metab 2003, 14: 56–7.
Wiedermann CJ. Secretoneurin: a functional neuropeptide in health and disease. Peptides 2000, 21: 1289–98.
Kirchmair R, Benzer A, Troger J, et al. Molecular characterization of immunoreactivities of peptides derived from chromogranin A (GE-25) and from secretogranin II (secretoneurin) in human and bovine cerebrospinal fluid. Neuroscience 1994, 63: 1179–87.
Deftos LJ, Abrahamsson PA. Granins and prostate cancer. Urology 1998, 51: 141–5.
Yon L, Guillemot J, Montero-Hadjadje M, et al. Identification of the secretogranin II-derived peptide EM66 in pheochromocytomas as a potential marker for discriminating benign versus malignant tumors. J Clin Endocrinol Metab 2003, 88: 2579–85.
Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 1992, 257: 967–71.
Peinado JR, Castano JP, Vazquez-Martinez R, et al. Amphibian melanotrophs as a model to analyze the secretory plasticity of endocrine cells. Gen Comp Endocrinol 2002, 126: 4–6.
Nickel W, Brugger B, Wieland FT. Protein and lipid sorting between the endoplasmic reticulum and the Golgi complex. Semin Cell Dev Biol 1998, 9: 493–501.
Barlowe C. COPII: a membrane coat that forms endoplasmic reticulum-derived vesicles. FEBS Lett 1995, 369: 93–6.
Barlowe C. COPII and selective export from the endoplasmic reticulum. Biochim Biophys Acta 1998, 1404: 67–76.
Dominguez M, Dejgaard K, Fullekrug J, et al. gp25L/emp24/ p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol 1998, 140: 751–65.
Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001, 2: 107–17.
Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol 2004, 16: 451–7.
Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol 2001, 313: 889–901.
Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol 2001, 13: 500–11.
Chavrier P, van der Sluijs P, Mishal Z, et al. Early endosome membrane dynamics characterized by flow cytometry. Cytometry 1997, 29: 41–9.
Mohrmann K, van der Sluijs P. Regulation of membrane transport through the endocytic pathway by rab GTPases. Mol Membr Biol 1999, 16: 81–7.
Pind SN, Nuoffer C, McCaffery JM, et al. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol 1994, 125: 239–52.
Nuoffer C, Davidson HW, Matteson J, et al. A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol 1994, 125: 225–37.
Vitelli R, Santillo M, Lattero D, et al. Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem 1997, 272: 4391–7.
Riederer MA, Soldati T, Shapiro AD, et al. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol 1994, 125: 573–82.
Fischer von Mollard G, Stahl B, Khokhlatchev A, et al. Rab3C is a synaptic vesicle protein that dissociates from synaptic vesicles after stimulation of exocytosis. J Biol Chem 1994, 269: 10971–4.
Stahl B, von Mollard GF, Walch-Solimena C, et al. GTP cleavage by the small GTP-binding protein Rab3A is associated with exocytosis of synaptic vesicles induced by α-latrotoxin. J Biol Chem 1994, 269: 24770–6.
Lledo PM, Vernier P, Vincent JD, et al. Inhibition of Rab3B expression attenuates Ca(2+)-dependent exocytosis in rat anterior pituitary cells. Nature 1993, 364: 540–4.
Lledo PM, Johannes L, Vernier P, et al. Rab3 proteins: key players in the control of exocytosis. Trends Neurosci 1994, 17: 426–32.
Johannes L, Lledo PM, Roa M, et al. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J 1994, 13: 2029–37.
Holz RW, Brondyk WH, Senter RA, et al. Evidence for the involvement of Rab3A in Ca(2+)-dependent exocytosis from adrenal chromaffin cells. J Biol Chem 1994, 269: 10229–34.
Regazzi R, Ravazzola M, Iezzi M, et al. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci 1996, 109: 2265–73.
Waselle L, Coppola T, Fukuda M, et al. Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell 2003, 14: 4103–13.
Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med 2002, 8: 23–30.
Lutcke A, Parton RG, Murphy C, et al. Cloning and subcel-lular localization of novel rab proteins reveals polarized and cell type-specific expression. J Cell Sci 1994, 107: 3437–48.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vàzquez-Martínez, R., Peinado, J.R., Cruz-García, D. et al. Melanotrope cells as a model to understand the (patho)physiological regulation of hormone secretion. J Endocrinol Invest 28, 949–958 (2005). https://doi.org/10.1007/BF03345330
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345330