Abstract
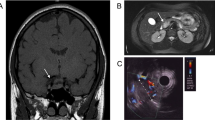
Multiple endocrine neoplasm type 1 (MEN1) syndrome predisposes to the development of endocrine and non-endocrine tumors with an autosomal dominant pattern of inheritance. Different mutations have been found throughout the gene with a variable phenotype expression. The proband, a Caucasian man, was admitted to our department in 2001, at the age of 51 because of a 1-yr history of diarrhoea and hypertension. He reported a previous intestinal resection for bowel occlusion with a histological diagnosis of unspecified mesenchymal neoplasia. He had also undergone a left adrenalectomy for a large non-functioning adrenal adenoma. Subsequently, he had suffered from gastralgia and melena; a gastroduodenoscopy showed an erosive gastritis. His family history was negative for endocrine disorders. On physical examination, multiple abdominal cutaneous lipomas and facial angiofibromas were observed. Biochemical screening revealed a primary hyperparathyroidism and an increase in circulating levels of PRL, chromogranin-A, gastrin and glucagon. The whole body computed tomography (CT) scan, the 111In-octreotide scan and the pituitary magnetic resonance imaging (MRI) did not reveal any abnormality. The presence of small neuroendocrine tumors was suspected by a positron emission tomography uptake in the epigastric region. The endoscopic ultrasound revealed a pancreatic lesion sized 1.1 cm that is under evaluation. Direct DNA sequencing analysis of the proband MEN1 gene revealed the 579delG frameshift mutation in the exon 3. The genetic screening of the family revealed the same mutation in 3 out of 5 offspring. The biochemical screening revealed some features of the MEN1 syndrome in all three of them. In conclusion, a novel frameshift MEN1 mutation was found in kindred with an apparently negative family history. Our experience confirms that MEN1 syndrome is a complex and underestimated condition, unless specifically investigated by trained specialists.
Similar content being viewed by others
References
Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 2001, 86: 5658–71.
Schussheim DH, Skarulis MC, Agarwal SK, et al. Multiple endocrine neoplasia type 1: new clinical and basic findings. Trends Endocrinol Metab 2001, 12: 173–8.
Wermer P. Genetic aspects of adenomatosis of endocrine glands. Am J Med 1954, 16: 363–71.
Wermer P. Endocrine adenomatosis, peptic ulcer disease in a large kindered: inherited multiple tumors, mosaic pleiotropism in man. Am J Med 1963, 35: 205–8.
Larsson C, Skogseid B, Oberg K Nakamura Y, Nordenskjold M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature 1988, 332: 85–7.
Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the genefor multiple endocrine neoplasia-type 1. Science 1997, 276: 404–7.
Agarwal SK, Lee Burns A, Sukhodolets KE, et al. Molecular pathology of the MEN1 gene. Ann NY Acad Sci 2004, 1014: 189–98.
Jin S, Mao H, Schnepp RW, et al. Menin associates with FANCD2, a protein involved in repair of DNA damage. Cancer Res 2003, 63: 4204–10.
Kim H, Lee JE, Cho EJ, et al. Menin, a tumor suppressor, represses JunD mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res 2003, 63: 6135–9.
Schnepp RW, Hou Z, Wang H, et al. Functional interaction between tumor suppressor menin and activator of S-phase kinase. Cancer Res 2004, 64: 6791–6.
Sowa H, Kaji H, Hendy GN, et al. Menin is required for bone morphogenetic protein 2- and transforming growth factor beta-regulated osteoblastic differentiation through interaction with Smads and Runx 2. J Biol Chem 2004, 279: 40267–75.
Agarwal SK, Kester MB, Debelenko LV, et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Genet 1997, 6: 1169–75.
Brandi ML. Multiple endocrine neoplasia type 1. Rev Endocr Metab Disord 2000, 1: 275–82.
Schussheim DH, Skarulis MC, Agarwal SK, et al. Multiple endocrine neoplasia type 1: new clinical and basicfindings. Trends Endocrinol Metab 2001, 12: 173–8.
Glascock MJ, Carty SE. Multiple endocrine neoplasia type 1: fresh perspective on clinical features and penetrance. Surg Oncol 2002, 11: 143–50.
Ferolla P, Falchetti A, Filosso P, et al. Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. J Clin Endocrinol Metab 2005, 90: 2603–8.
Trump D, Farren B, Wooding C, et al. Clinical studies of multiple endocrine neoplasia type I. Q J Med 1996, 89: 653–69.
Gibril F, Venzon DJ, Ojeaburu JV. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab 2001, 86: 5282–93.
Skogseid B, Laesson C, Lindgren PG, et al. Clinical and genetic features of adrenocortical lesions in multiple endocrine neoplasia type-1. J Clin Endocrinol Metab 1992, 75: 76–81.
Langer P, Cupisti K, Bartsch DK, et al. Adrenal involvement in multiple endocrine neoplasia type 1. World J Surg 2002, 26: 891–6.
Barzon L, Pasquali C, Grigoletto C, Pedrazzoli S, Boscaro M, Fallo F. Multiple endocrine neoplasia type 1 and adrenal lesions. J Urol 2001, 166: 24–7.
Burgess JR, Shepherd JJ, Parameswaran V, et al. Spectrum of pituitary disease in multiple endocrine neoplasia type 1 (MEN1): clinical, biochemical and radiological features of pituitary disease in a large MEN1 kindred. J Clin Endocrinol Metab 1996, 81: 2642–6.
Kaltsas GA, Bessere M, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 2004, 25: 458–511.
Lamberts SWJ, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev 1991, 12: 450–82.
Kwekkeboom DJ, Krenning EP. Somatostatin receptors imaging. In: Lamberts SWJ, Dogliotti L eds. The expanding role of octreotide I: advances in oncology. Bristol: BioScientifica Ltd. 2002, 17–30.
Beckers A, Betea D, ValdesSocin H, Stevenaert A. The treatment of sporadic versus MEN-I related pituitary adenomas. J Intern Med 2003, 253: 599–605.
Verges B, Boureille F, Goudet P, et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multi-center study. J Clin Endocrinol Metab 2002, 87: 457–65.
Shi W, Buchanan KD, Johnston CF, et al. The octreoscan suppression test and [111 In-DTPA-D-Phe1]-octreotide scintigraphy in neuroendocrine tumours correlate with responsiveness to somatostatin analogue treatment. Clin Endocrinol (Oxf) 1998, 48: 303–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nuzzo, V., Tauchmanová, L., Falchetti, A. et al. MEN1 family with a novel frameshift mutation. J Endocrinol Invest 29, 450–456 (2006). https://doi.org/10.1007/BF03344129
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03344129