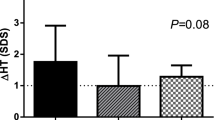
Abstract
Serum GH levels were measured in 9 prepubertal children with growth hormone (GH) deficiency using an immunofluorometric assay (IFMA) and a Nb2 cell bioassay, prior to and 2, 4, 6 and 12 hours after the first hGH subcutaneous injection (sc) (0.1 IU/Kg). After this first acute phase, hGH treatment was continued regularly at a dose of 0.1 IU/kg per day at bedtime. The acute pharmacokinetic profile was similar in the patients irrespectively of the assay used: serum GH usually starts to rise after 2 hours, peaks by 4 or 6 hours and drops back to near baseline levels 12 hours after sc GH administration. A great variation in the amplitude of peak levels was observed among the patients. The assays significantly (p<0.0001) correlate with each other in all subjects. An increase in serum GH values as evaluated by the Nb2 bioassay was observed in most children after sc GH injection, suggesting that the administered hormone preserves its biological activity. The individual variations in GH bioactivity in our GH-deficient children could be due to different mechanisms of the drug’s degradation at the site of injection or in circulation. Pre-treatment height velocity calculated as standard deviation scores (SDS) significantly increased (p<0.001) from -2.77±0.42 to 1.22±0.87 SDS after a 12-month period of GH treatment in 8 patients who had already completed the first year of therapy, although it varied considerably among subjects. A higher serum GH peak value evaluated by both assays corresponded to a higher growth response during the 1st year, but not in the 2nd year of GH therapy. In conclusion, the degree of GH bioactivity released into circulation after sc GH injection may vary considerably among GHD patients and correlates with the growth response observed during the 1st year of GH therapy.
Similar content being viewed by others
References
Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Guidelines for the use of growth hormone in children with short stature. J. Pediatr. 1995, 127: 857–867.
Fraiser S.D., Costin G., Lippe B.M., Aceto T., Bunger P.F. A dose-response curve for human growth hormone. J. Clin. Endocrinol. Metab. 1981, 53: 1213–1217.
Frasier S.D. Human pituitary growth hormone (hGH) therapy in growth hormone deficiency. Endocr. Rev. 1983, 4: 155–170.
Preece M.A., Tanner J.M., Whitehouse R.H., Cameron N. Dose dependence of growth response to human growth hormone in growth hormone deficiency. J. Clin. Endocrinol. Metab. 1976, 42: 477–483.
Ranke M., Weber B., Bierich J.R. Long-term response to human growth hormone in 36 children with idiopathic growth hormone deficiency. Eur. J. Pediatr. 1979, 132: 221–238.
Job J.C., Chaussain J.L., Garnier P., Rolland A., Joab N. Dose-response relationship in the treatment of hypopituitary children with human growth hormone: a retrospective survey. Acta Paediatr. Scand. 1987, 337(Suppl.): 93–105.
Kastrup K.W., Christiansen S.J., Andersen K.J., Onskov H. Increased growth rate following transfer to daily subcutaneous administration from three weekly intramuscular injections of hGH in growth hormone deficient children. Acta Endocrinol. 1983, 104: 148–152.
Greulich W., Pyle S.I. Radiographic Atlas of Skeletal Development of the Hand and Wrist, ed. 2. Stanford University Press, Palo Alto, 1959.
Albertsson-Wikland K., Jansson C., Rosberg S., Novamo A. Time-resolved immunofluorometric assay of human growth hormone. Clin. Chem. 1993, 39: 1620–1625.
Rott A.W., Duckett G.E., Geiszler J.E., Hu C.S., Bercu B.B. Evaluation of the clinical utility of the ultrasensitive immunofluorometric assay for growth hormone (GH) and of the cortisol secretory pattern in prediction of the linear growth response to treatment with GH. J. Pediatr. Endocrinol. Metab. 1997, 13: 3–10.
Tanaka T., Shiu R.P.C., Gout P.W., Beer C.T., Noble R.L., Friesen H.G. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J. Clin. Endocrinol. Metab. 1980, 51: 1058–1063.
Walker A., Croze F., Friesen H.G. A serum-free medium for culturing lactogen dependent and autonomous Nb2 node lymphoma cells. Endocrinology 1987, 120: 2389–2397.
Bozzola M., Zecca M., Locatelli F., Radetti R., Pagani S., Autelli M., Tatò L., Chatelain P. Evaluation of growth hormone bioactivity using the Nb2 cell bioassay in children with growth disorders. J. Endocrinol. Invest. 1998, 21: 765–770.
Hintz R.L., Rosenfeld R.G., Wilson D.M., Bennet A., Finno J., McClellan B., Swify R. Biosynthetic methionyl human growth hormone is biologically active in adult man. Lancet 1982, i: 276–279.
Takano K., Hizuka N., Shizume K., Asakawa K., Kogawa M. Short-term study of biosynthesised hGH in man. Endocrinol. Jpn. 1983, 30: 79–84.
Takano K., Shizume K. Current clinical trials with authentic recombinant human growth hormone in Japan. Acta Paediatr. Scand. 1986, 325(Suppl.): 93–97.
Wilton P., Sietnieks A. An open-labelled study of the safety acute metabolic activity and pharmacokinetic profile of a short-term course of recombinant human growth hormone in healthy volunteers. Clin. Endocrinol. (Oxf.) 1987, 26: 125–128.
Usala A.L., Blumer J.L. Pharmacology of new hormonal therapies in the treatment of paediatric endocrine disorders. Pediatr. Clin. North Am. 1989, 36: 1157–1182.
Moore J.A., Rudman C.G., McLachlan N.J., Fuller G.B., Burnett B., Frane J.W. Equivalent potency and pharmacokinetics of recombinant human growth hormones with or without an N-terminal methionine. Endocrinology 1988, 122: 2920–2926.
Levin P., Chalew S., Martin L., Kowarski A. Comparison of assays for growth hormone using monoclonal or polyclonal antibodies for diagnosis of growth disorders. J. Lab. Clin. Med. 1987, 109: 85–88.
Woodhead S., Turner G. Accuracy of growth hormone measurements. Horm. Res. 1991, 36(Suppl.): 17–20.
Albertsson-Wikland K. The effect of human growth hormone injection frequency on linear growth rates. Acta Paediatr. Scand. 1987, 337(Suppl.): 110–116.
Jorgensen J.O.L., Moller N., Lauritzen T., Christiansen J.S. The absorption kinetics of subcutaneously administered growth hormone: influence of injection volume. J. Pediatr. Endocrinol. 1989, 3: 213–215.
Christiansen J.S., Orskov H., Binder C., Kastrup K.W. Imitation of normal plasma growth hormone profile by subcutaneous administration of human growth hormone to growth hormone deficient children. Acta Endocrinol. (Copenh.) 1983, 102: 6–10.
Houdijik E.C.A.M., Herdes E., Delemarre-Van de Waal H.A. Pharmacokinetics and pharmacodynamics of recombinant human growth hormone by subcutaneous jet- or needle-injection in patients with growth hormone deficiency. Acta Paediatr. 1997, 86: 1301–1307.
Baumann G. Heterogeneity of growth hormone. In: Bercu B.B., (ed.) Basic and clinical aspects of growth hormone. Plenum Press, New York, 1988, pp. 13–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bozzola, M., Radetti, G., Pagani, S. et al. The level of bioavailable growth hormone (GH) after the first GH injection predicts the first year’s growth response in GH-deficient children. J Endocrinol Invest 22, 790–795 (1999). https://doi.org/10.1007/BF03343645
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03343645