Abstract
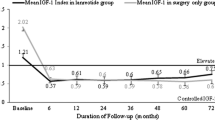
Objective: To assess the efficacy and tolerability of SR-lanreotide in the treatment of active acromegaly. Patients and design: 30 patients (17 men and 13 women) were treated in whom active acromegaly was confirmed by clinical features, a mean GH level of >5 mlU/l and failure to suppress GH to <2 mIU/l after a 75 g glucose load. Patients were treated for a median period of 60 weeks (range 12-168) with im injections of SR-lanreotide 30 mg given every 7-14 days. Measurements: Mean GH and IGF-I levels were measured at baseline and every 12-weeks together with symptom score assessment. MRI of the pituitary gland was performed at baseline and if an adenoma was identified at yearly intervals. Gall bladder ultrasound scans were performed at baseline and then every 24-weeks. Results: Twenty-three patients were treated for at least 48-weeks and, in these, GH levels fell from 10.5 mlU/l (7.6-17.6) (median and interquartile range) at baseline to 3.2 mIU/l (2.4-3.9) (p<0.0001) and IGF-I levels ftom 88.9 nmol/L (71.4-137.1) to 56.8 nmol/l (39.3-75.4) (p=0.0002). GH response to treatment was better in elderly patients (age≥65 years) compared to younger patients but neither sex, pre-treatment GH levels, previous surgery nor previous radiotherapy influenced the response. Treatment resulted in a significant improvement in the symptoms of active acromegaly in the majority of patients. A significant reduction in the size of the pituitary adenoma was documented in 6 of 10 patients who had a repeat MRI scan after one year. Treatment was well-tolerated by the majority of patients; side effects were mainly transient gastrointestinal symptoms. These were severe in only 2 patients necessitating discontinuation of the drug. Two patients developed new gall stones and 4 female patients had temporal hair loss necessitating stopping treatment in one of them. There were minor effects on glucose tolerance which were not of clinical importance. Conclusion: Long-term treatment of acromegaly with SR-lanreotide is effective in controlling GH and IGF-l levels and symptoms and is well tolerated in the majority of patients.
Similar content being viewed by others
References
Melmed S. Acromegaly. N. Engl. J. Med. 1990, 322: 966–977.
Vance M.L., Harris A.G. Long-term treatment of 189 acromegalic patients with the somatostatin analogue octreotide, results of the international multicentre acromegaly study group. Arch. Intern. Med. 1991, 151: 1573–1578.
Newman C.B., Melmed S., Snyder P.J., Young W.F., Boyajy L.D., Levy R., Stewart W.N., Klibanski A., Molitch M., Gagel R.F., Boyd A.E., Sheeler L., Cook D., Malarkey W.B., Jackson I.M.D., Vance M.L., Thorner M.O., Ho P.J., Jaffe C.A., Frohman L.A., Kleinberg D.L. Safety and efficacy of long term octreotide therapy of acromegaly: results of a multicentre trial in 103 patients. J. Clin. Endocrinol. Metab. 1995, 80: 2768–2775.
Stewart P.M., Kane K.F., Stewart S.E., Lancranjan I., Sheppard M.C. Depot long-Acting somatostatin analog (Sandostatin-LAR) is an effective treatment for acromegaly. J. Clin. Endocrinol. Metab. 1995, 80: 3267–3272.
Fløgusted A.K., Halse J., Bakke S. Sandostatin LAR in acromegalic patients: Long term treatment. J. Clin. Endocrinol. Metab. 1997, 82: 23–28.
Boucekkine C., Catus F., Blumberg-Tick J., Pholsena M., Chanson P., Schaison G. Treatment of acromegaly by the slow-release formulation of a new somatostatin analogue, lanreotide. Ann. Endocrinol. (Paris) 1994, 55: 261–269.
Al-Maskari M., Gebbie J., Kendall-Taylor P. The effect of a new slow-release, long-acting somatostatin analogue, lanreotide in acromegaly. Clin. Endocrinol. (Oxf.) 1996, 45: 415–421.
Guisti M., Gussoni G., Cuttica C.M., Giordano G., the Italian multicentre slow-release lanreotide study group. Effectiveness and tolerability of slow release lanreotide treatment in active acromegaly: Six month report on an italian multicentre study. J. Clin. Endocrinol. Metab. 1996, 81: 2089–2097.
Caron P., Morange-Ramos I., Cogne M., Jaquet P. three year follow-up of acromegalic patients treated with intamuscular slow-release lanreotide. J. Clin. Endocrinol. Metab. 1997, 82: 18–22.
Pringle P.J., Jones J., Hindmarsh P.C., Preece M.A., Brook C.G.D. Performance of proficiency survey samples in two immunoradiometric assays of human growth hormone and comparison with patients’ samples. Clin. Chem. 1992, 38: 553–557.
Morrell D.J., Dadi H., More J., Taylor A.M., Dabestani A., Buchanan C.R., Holder A.T., Preece M.A. A monoclonal antibody to human insulin-like growth factor-I. Characterization, use in radio immunoassay and effect on the biological activities of the growth factor. J. Mol. Endocrinol. 1989, 2: 201–206.
van der Lely A.J., Harris A.G., Lamberts S.W.J. The sensitivity of growth hormone secretion to medical treatment in acromegalic patients: influence of age and sex. Clin. Endocrinol. (Oxf.) 1992, 37: 181–185.
Reubi J.C., Landolt A.M. The growth hormone responses to octreotide in acromegaly correlate with adenoma somatostatin receptor status. J. Clin. Endocrinol. Metab. 1989, 68: 844–850.
Lamberts S. W.J., van der Lelly A.J., de Herder W. W., Hofland L.J. Octreotide. N. Engl. J. Med. 1996, 334: 246–254.
Hofiand L.J., Lamberts S.W.I Somatostatin receptors and disease: role of receptor subtypes. In: Sheppard M.C., Franklyn J.A. (Eds.), Bailliere’s Clinical Endocrinology and Metabolism. W.B. Saunders Company Ltd., London, 1996, vol. 10., p. 163.
Frohman L.A. Editorial: Acromegaly: What constitutes optimal therapy? J. Clin. Endocrinol. Metab. 1996, 81: 443–445.
Bates A.S., Van’t Hoff W., Jones J.M., Clayton R.N. An audit of outcome of treatment in acromegaly. Q. I Med. 1993, 86: 293–299.
Mclellan A.R., Connel J.M., Beastell G.H., Teasdale G., Davies D.L. Growth hormone, body composition and somatomedin C after treatment of acromegaly. Q.J.M. 1988. 69: 997–1008.
Rajasoorya C., Holdaway I.M., Wrightson P., Scott D.J., Ibbertson H.K. Determinants of clinical outcome and survival in acromegaly. Clin. Endocrinol. (Oxf.) 1994, 41: 95–102.
Bates A.S., Evans A.J., Jones P. Assessment of GH status in acromegaly using serum growth hormone, serum insulin-like growth factor-I and urinary growth hormone levels. Clin. Endocrinol. (Oxf.) 1995, 42: 417–423.
Kuhn J.M., Basin C., Mollard M., de rough B., Obach R., Tolis G. Pharmacokinetic study and effect on growth hormone secretion in healthy volunteers of the new somastotatin analogue BIM 23014. Eur. J. Clin. Pharmacol. 1993, 45: 73–77.
Heron I., Thomas F., Dero M. Pharmacokinetics and efficacy of a long-acting formulation of the new somatostatin analogue BIM 23014 in patients with acromegaly. J. Clin. Endocrinol. Metab. 1993, 76: 721–727.
Johnson M. R., Chowdery H.S., Grint C., Lightman S.L. Pharmacokinetics and efficacy of the long-acting somatostatin analogue somatuline in acromegaly. Eur. J. Endocrinol. 1994, 130: 229–234.
Acromegaly therapy consensus development panel. Consensus statement: benefits versus risks of medical therapy for acromegaly. Am. J. Med. 1994, 97: 468–473.
Montini M., Gianola D., Pagani M.D., Pedroncelli A., Caldara R., Gherardi M., Bonelli M., Lancranjan I., Pagani G. Cholelithiasis and acromegaly: therapeutic strategies. Clin. Endocrinol. (Oxf.) 1994, 40: 401–406.
Harris A. G., Prestele H., Herold K., Boerlin V. Long-term efficacy of Sandostatin (SMS 201-995) in 178 acromegalic patients: results of the international multicentre acromegaly study group. In. Lamberts SM1 (Ed.), Sandostatin in the treatment of acromegaly Springer, Berlin, 1988, p. 117–125.
James R. A., Moller N., Chaterjee M., White M., Kendall-Taylor P. Carbohydrate tolerance and serum lipids in acromegaly before and during treatment with high dose octreotide. Diabet. Med. 1991, 8: 517–523.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Suliman, M., Jenkins, R., Ross, R. et al. Long-term treatment of acromegaly with the somatostatin analogue SR-lanreotide. J Endocrinol Invest 22, 409–418 (1999). https://doi.org/10.1007/BF03343583
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03343583