Abstract
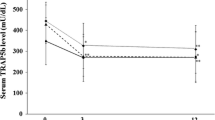
Recent animal work suggests that gamma-linolenic acid (GLA) and eicosapentaenoic acid (EPA) enhance calcium absorption, reduce excretion and increase calcium deposition in bone. A pilot study was set up to test the interactions between calcium and GLA+EPA in humans. Sixty-five women (mean age 79.5), taking a background diet low in calcium, were randomly assigned to GLA+EPA or coconut oil placebo capsules; in addition, all received 600 mg/day calcium as the carbonate. Markers of bone formation/degradation and bone mineral density (BMD) were measured at baseline, 6, 12 and 18 months. Twenty-one patients were continued on treatment for a second period of 18 months, after which BMD (36 months) was measured. At 18 months, osteocalcin and deoxypyridinoline levels fell significantly in both groups, indicating a decrease in bone turnover, whereas bone specific alkaline phosphatase rose indicating beneficial effects of calcium given to all the patients. Lumbar and femoral BMD, in contrast, showed different effects in the two groups. Over the first 18 months, lumbar spine density remained the same in the treatment group, but decreased 3.2% in the placebo group. Femoral bone density increased 1.3% in the treatment group, but decreased 2.1% in the placebo group. During the second period of 18 months with all patients now on active treatment, lumbar spine density increased 3.1% in patients who remained on active treatment, and 2.3% in patients who switched from placebo to active treatment; femoral BMD in the latter group showed an increase of 4.7%. This pilot controlled study suggests that GLA and EPA have beneficial effects on bone in this group of elderly patients, and that they are safe to administer for prolonged periods of time.
Similar content being viewed by others
References
Heaney R.P.: Calcium in the prevention and treatment of osteoporosis. J. Intern. Med. 231: 169–180, 1992.
Chapuy M.C., Meunier P.J.: Prevention of secondary hyperparathyroidism and hip fracture in elderly women with calcium and vitamin D3 supplements. Osteoporos. Int. 3 (Suppl.): S60–S63, 1996.
Ledger G.A., Burritt M.F., Kao P.C., O’Fallon W.M., Riggs B.L.: Abnormalities of parathyroid hormone secretion in elderly women that are reversible by short-term therapy with 1,25 dihydroxy-vitamin D3. J. Clin. Endocrinol. Metab. 79 (1): 211–216, 1994.
Kochersberger G., Bales C., Lobough B., Lyles K.W.: Calcium supplementation lowers serum parathyroid hormone levels in elderly subjects. J. Gerontol. 45 (5): M159–M162, 1990.
Rasmussen H., Matsumoto T., Fontaine Q., Goodman D.B.P.: Role of changes in membrane lipid structure in the action of 1,25-dihydroxyvitamin D3. Fed. Proc. 41: 72–77, 1982.
Hay A.W.M., Hassam A.G., Crawford M., Stevens P.A., Maver E.B., Sutherland Jones F.: Essential fatty acid restriction inhibits vitamin D-dependent calcium absorption. Lipids 15 (4): 251–254, 1985.
Horrobin D.F.: Gammalinolenic acid: An intermediate in essential fatty acid metabolism with potential as an ethical pharmaceutical and as a food. Rev. Contemp. Pharmacother. 1: 1–45, 1990.
Meyer-Werger A., Jordan P., Moser U.K.: PUFA deficiency as risk factor for developing osteoporosis: results from the Seneca study. Int. Conference on highly unsaturated fatty acids in nutrition and disease prevention, Barcelona, Spain, 1996 (Abstract).
Borland V.G., Jackson C.M.: Effects of a fat free diet on the structure of the kidney in rats. Arch. Pathol. 11: 687–708, 1931.
Alfin-Slater R., Bernick S.: Changes in tissue lipids and tissue histology resulting from essential fatty acid deficiency in rats. Am. J. Clin. Nutr. 6: 613–624, 1958.
Sinclair H.M.: Deficiency of essential fatty acids in lower animals. In: Essential fatty acids. Butterworths, London, 1957, pp. 249–256.
Claassen N., Potgieter H.C., Seppa M., Vermaak W.J., Coetzer H., Van Papendorp D.H., Kruger M.C.: Supplemented gamma-linolenic acid and eicosapentaenoic acid influence bone status in young male rats: effects on free urinary collagen crosslinks, total urinary hydroxyproline and bone calcium content. Bone 16: 385S–392S, 1995.
Kruger M.C., Claassen N., Potgieter H.C., Coetzer H., de Winter R.: Essential fatty acid supplementation and calcium retention in the ovariectomised rat. Osteoporos. Int. 6 (1): 101, 1996.
Monsen E.R.: The 10th edition of the recommended dietary allowances: What’s new in the 1989 RDAs. J. Am. Diet. Assoc. 89 (12): 1748–1752, 1989.
Van Papendorp D.H., Coetzer H., Kruger M.C.: Biochemical profile of osteoporotic patients on fatty acid supplementation. Nutr. Res. 15 (3): 325–334, 1995.
Tietz N.W.: Clinical guide to laboratory tests, ed. 2. Saunders, Philadelphia, PA, 1990.
Mazess R., Collick B., Trempe J., Barden H., Hanson J.: Performance evaluation of a dual-energy X-ray bone densitometer. Calcif. Tissue Int. 44 (3): 228–232, 1989.
Melton J.L., Atkinson E.J., O’Fallon M., Wahner H.W., Riggs B.L.: Long-term fracture prediction by bone mineral assessed at different skeletal sites. J. Bone Miner. Res. 8 (10): 1227–1232, 1993.
Folch J., Lees M., Sloane-Stanley G.H.: A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 479–509, 1956.
Manku M.S., Horrobin D.F., Huang Y.S., Morse N.: Fatty acids in plasma and red cell membranes in normal humans. Lipids 18: 906–908, 1983.
Bell N.H.: Vitamin D metabolism, aging and bone loss. J. Clin. Endocrinol. Metab. 80 (4): 1051, 1995.
O’Doherty P.J.A.: Dihydroxyvitamin D3 increases the activity of the intestinal phosphatidylcholine deacylation — reacylation cycle. Lipids 14: 75–77, 1978.
Lau K., Longman C.B., Gafter U., Dudeja P.K., Brasitus T.: Increased calcium absorption in prehypertensive spontaneously hypertensive rats. Role of serum 1,25 dihydroxyvitamin D3 levels and intestinal brush border membrane fluidity. J. Clin. Invest. 78: 1083–1090, 1986.
Boyan B.D., Dean D.D., Sylvia V.L., Schwartz Z.: Non-genomic regulation of extracellular matrix events by vitamin D metabolites. J. Cell. Biochem. 56: 331–339, 1994.
Popp-Snyders C., Schouten J.A., De Jong A.P., Van der Veen E.A.: The effect of dietary cod-liver oil on the lipid composition of human erythrocyte membranes. Scand. J. Clin. Lab. Invest. 44: 39–46, 1984.
Kruger M.C., Coetzer H., de Winter R., Claassen N.: Eico -sapentaenoic acid and docosahexaenoic acid supplementation increases calcium balance. Nutr. Res. 15: 211–219, 1995.
Coetzer H., Claassen N., Van Papendorp D.H., Kruger M.C.: Calcium transport by isolated brush border and basolateral membrane vesicles. Role of essential fatty acid supplementation. Prostaglandins. Leukot. Essent. Fatty Acids 50: 251–266, 1994.
Yamada Y., Fushimi H., Inoue T., Matsuyama Y., Kameyama M., Minami T., Okazaki Y., Noguchi Y., Kasama T.: Effect of eicosapentaenoic acid and docosahexaenoic acid on diabetic osteopenia. Diabetes Res. Clin. Practice 30: 37–42, 1995.
Sakaguchi K., Murota S.: Eicosapentaenoic acid inhibits bone loss due to ovariectomy in rats. Prostaglandins. Leukot. Essent. Fatty Acids 50: 81–84, 1994.
Eastell R., Yergey A.L., Vieira N.E., Cedel S.L., Kumar R., Riggs B.L.: Interrelationship among vitamin D metabolism, true calcium absorption, parathyroid function and age in women: Evidence of an age-related intestinal resistance to 1,25-dihydroxyvitamin D action. J. Bone Miner. Res. 6 (2): 125–132, 1991.
Epstein S., Bryce G., Hinman J.W., Miller O.N., Riggs B.L., Hui S.L., Johnston C.C.: The influence of age on bone mineral regulating hormones. Bone 7: 421–425, 1986.
Mundy G.R.: Bone remodelling and its disorders. Martin Dunitz, London, 1995.
Caulfield M.P.: Biochemical markers of bone resorption. Endocrinology 13 (2): 47–55, 1995.
Kushida K., Takahashi M., Kawana K., Inoue T.: Comparison of markers for bone formation and resorption in premenopausal and postmenopausal subjects and osteoporosis patients. J. Clin. Endocrinol. Metab. 80 (8): 2447–2450, 1995.
Hassager C., Fabbri-Mabelli G., Christiansen C.: The effect of the menopause and hormone replacement therapy on serum carboxyterminal propeptide of type 1 collagen. Osteoporos. Int. 3: 50–52, 1993.
Charles P., Mosekilde L., Risteli L., Risteli J., Eriksen E.F.: Assessment of bone remodeling using biochemical indicators of type I collagen synthesis and degradation: relation to calcium kinetics. Bone Miner. 24: 81–94, 1994.
Reid I.R., Ames R.W., Evans M., Gamble G.D., Sharpe S.J.: Effect of calcium supplementation on bone loss in postmenopausal women. N. Engl. J. Med. 328 (7): 460–464, 1993.
Eriksen E.F., Charles P., Melsen F., Mosekilde L., Risteli L., Risteli J.: Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. J. Bone Miner. Res. 8 (2): 127–132, 1993.
Aloia J.F., Vaswani A., Yeh J.K., Ross P.L., Flaster E., Dilmanian F.A.: Calcium supplementation with and without hormone replacement therapy to prevent postmenopausal bone loss. Ann. Intern. Med. 120 (2): 97–103, 1994.
Keen R.W., Nguyen T., Sobnack R., Perry L.A., Thompson P.W., Spector T.D.: Can biochemical markers predict bone loss at the hip and spine? A 4-year prospective study of 141 early postmenopausal women. Osteoporos. Int. 6: 399–406, 1996.
Kanis J.A.: Calcium nutrition and its implications for osteoporosis. Part ll. After menopause. Eur. J. Clin. Nutr. 48: 833–841, 1994.
Heany R.P.: Interpreting trials with bone-active agents. Am. J. Med. 98: 329–330, 1995.
Baggio B., Gambaro G., Zambon S., Marchini F., Bassi A., Bordin L., Clarl G., Manzato E.: Anomalous phospholipid n-6 polyunsaturated fatty acid composition in idiopathic calcium nephrolithiasis. J. Am. Soc. Nephrol. 7 (4): 613–620, 1996.
Buck A.C., Davies R.L., Harrison T.: The protective role of eicosapentaenoic acid in the pathogenesis of nephrolithiasis. J. Urol. 146: 188–194, 1991.
Raisz L.G., Pilbeam C.C., Fall P.M.: Prostaglandins: Mechanism of action and regulation of production in bone. Osteoporos. Int. 1 (Suppl.): S136–S140, 1993.
Watkins B.A., Shen C-L., McMurtry J.P., Xu H., Allen K.G.D., Seifert M.F.: Dietary lipids modulate bone prostaglandin E2 production, insulin-like growth factor-1 concentration and formation in chicks. J. Nutr. 127: 1084–1091, 1997.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kruger, M.C., Coetzer, H., de Winter, R. et al. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging Clin Exp Res 10, 385–394 (1998). https://doi.org/10.1007/BF03339885
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03339885