Abstract
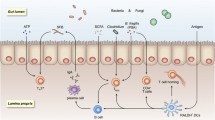
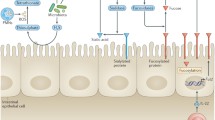
Based on Pasteur’s work on the microbial nature of fermentation, it was widely believed that the presence of bacteria in the intestine was essential for the life of the host. It has also been known for decades that gut commensal microbes effect the activation and development of the systemic immune system through gutassociated lymphoid tissues (GALT). Recent extensive studies have shown that recognition of microbes is mediated by a set of germline-encoded receptors, Toll-like receptors (TLRs), in mammals. This article reviews the role of the innate immunity system in the development of GALT and the pathogenesis of inflammatory bowel diseases (IBD).
Similar content being viewed by others
References
Tlaskalova H, Sterzl J, Hajek P, Pospisol M, Riha I, Marvanova H, et al. The development of antibody formation during embryonal and postnatal periods. In: Sterzl J, Riha I, editors. Development aspects of antibody formation and structure. Prague: Academic Publishing House; 1970. p. 767–90.
Carter PB, Pollard M. Host responses to “normal” microbial flora in germ-free mice. J Reticuloendothelial Soc 1971;9:580–7.
Berg RD, Savage DC. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun 1975;11:320–9.
Shroff KE, Meslin K, Cebra JJ. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun 1995;63:3904–13.
Cebra JJ, Jiang HQ, Sterzl J, Tlasklova-Hogenova H. The role of mucosal microbiota in the development and maintenance of the mucosal immune system. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal immunology. San Diego: Academic; 1999. p. 266–80.
De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 1994;264: 703–7.
Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, et al. Lymphotoxin-a-deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol 1995; 155:1685–93.
Alimzhanov MB, Kuprashi DV, Kosco-Vibois MH, Luz A, Turetskaya RL, Tarakhovsky A, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin b-deficient mice. Proc Natl Acad Sci USA 1997;94:9302–7.
Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, et al. Lymphotoxin-b, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 1993;72:847–56.
Glimcher LH. Lineage commitment in lymphocytes: controlling the immune response. J Clin Invest 2001;108:25–30.
Guy-Grand D, Griscelli C, Vassalli F. Peyer’s patches, gut IgA plasma cells and thymic function: study in nude mice bearing thymic grafts. J Immunol 1975;115:361–4.
Craig SW, Cebra JJ. Peyer’s patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med 1971;134:188–200.
Elson CO, Heck JA, Strober W. T-cell regulation of murine IgA synthesis. J Exp Med 1979;149:632–43.
Schultz CL, Coffman RL. Control of isotype switching by T cells and cytokines. Curr Opin Immunol 1991;3:350–4.
Coffman RL, Lebman DA, Shrader B. Transforming growth factor b specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med 1989;170:1039–44.
Dubois B, Bridon JM, Fayette J, Barthelemy C, Banchereau J, Caux C, et al. Dendritic cells directly modulate B cell growth and differentiation. J Leukocyte Biol 1999;66:224–30.
Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today 1997;18:335–43.
Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today 1998;19:173–81.
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001;2:675–80.
Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996;86:973–83.
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997;388:394–7.
Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drodophila Toll. Proc Natl Acad Sci USA 1998;95:588–93.
Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem 1999;274:17406–9.
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda K, et al. Cutting edge. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 1999;162:3749–52.
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. Toll-like receptor recognizes bacterial DNA. Nature2000;408: 740–5.
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor-5. Nature 2001;410:1099–103.
Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol 2002;168:554–61.
Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor-and MyD88-knockout mice. Trends Immunol 2001;22:78–83.
Adachi O, Kawai T, Takeda K, Tsutsui H, Sakagami M, Nakanishi K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 1998;9: 143–50.
MacDonald TT, Monteleone G. IL-12 and Thl immune responses in human Peyer’s patches. Trends Immunol 2001;22:244–7.
Nagata S, McKenzie C, Pender SLF, Bajaj-Elliott M, Fairclough PD, Walker-Smith JA, et al. Human Peyer’s patch T cells are sensitized to dietary antigen and display a Th cell type 1 cytokine profile. J Immunol 2000;165:5315–21.
Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, et al. IL-7 receptor α+ CD3- cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int Immunol 1999;11:643–55.
Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology 1984;86:174–93.
Duchmann R, Neurath MF, Meyer zum Buschenfelde KH. Responses to self and non-self intestinal microflora in health and inflammatory bowel disease. Res Immunol 1997;148:589–94.
Lebman DA, Edmiston JS. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect 1999;l:1297–304.
Ehrhardt RO, Ludviksson BR, Gray B, Neurath M, Strober W. Induction and prevention of colonic inflammation in IL-2-deficient mice. J Immunol 1997;158:566–73.
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993;75:263–74.
Duchmann R, Schmitt E, Knolle P, Meyer zum Buschenfelde KH, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol 1996;26:934–8.
Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 1993;75:275–82.
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora, but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995;102:448–55.
Duchmann R, May E, Heike M, Knolle P, Neurath M, Meyer zum Buschenfelde KH. T cell specificity and cross reactivity towards enterobacteria, Bacteroides, Bifidobacterium, and antigens from resident intestinal flora in humans. Gut 1999;44:812–8.
Macpherson A, Khoo UY, Forgacs I, Philport-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 1996;38:365–75.
Hollander D. Permeability in Crohn’s disease: altered barrier function in healthy relatives? Gastroenterology 1993; 104:1848–51.
Satsangi J, Jewell DP, Rosenberg WM, Bell JI. Genetics of inflammatory bowel disease. Gut 1994;35:696–700.
Podolsky DK. Inflammatory bowel disease (first of two). N Engl J Med 1995;325:928–37.
Fiocchi C. Inflammatory bowel disease—etiology and pathogenesis. Gastroenterology 1998;115:182–205.
Fuss IJ, Neurath M, Boirvant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 1996;157:1261–70.
Parronchi P, Romagnani P, Annuziato F, Sampognaro S, Becchio A, Giannarini L, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn’s disease. Am J Pathol 1997;150:823–32.
Kanai T, Watanabe M, Okazawa A, Sato T, Yamazaki M, Okamoto S, et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn’s disease. Gastroenterology 2001;121:875–88.
Lang KA, Peppercorn MA. Medical therapy for Crohn’s disease. In: Kirsner JB, editors. Inflammatory bowel disease. Philadelphia: W.B. Saunders; 2000. pp. 557–77.
Colombel JF, van Kruiningen HJ. Antibiotics in Crohn’s disease. Gut 2001;48:647–8.
Bernstein LH, Frank MS, Brandt LJ, Boley SJ. Healing of perineal Crohn’s disease with metronidazole. Gastroenterology 1980;79:357–65.
Prantera C, Zannoni F, Scribano ML, Berto E, Andredi A, Kohn A, et al. An antibiotics regimen for the treatment of active Crohn’s disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 1996;91:328–32.
Colombel JF, Lemann M, Cassagnou M, Bouhnik Y, Duclos B, Dupas JL, et al. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn’s disease. Am J Gastroenterol 1999;94:674–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kanai, T., Iiyama, R., Ishikura, T. et al. Role of the innate immune system in the development of chronic colitis. J Gastroenterol 37 (Suppl 14), 38–42 (2002). https://doi.org/10.1007/BF03326411
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03326411