Abstract
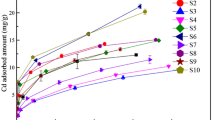
Lead is one of the most dangerous contaminants which has been released to the environment during many years by anthropogenic activities. Adsorption of Pb2+ on vermicompost was studied at 11°C, 30 °C and 50 °C by using Langmuir and Freundlich models, that adequately described the adsorption process, with maximum adsorption capacities were 116.3; 113.6 and 123.5 μg/g for each temperature. The differences in FTIR (Fourier Transform Infrared Spectrometer) spectra of vermicompost at pH 3,8 and pH 7.0 in the region from 1800 to 1300 cm-1 were interpreted on the basis of carboxyl acid ionization that reduce band intensity around 1725 cm-1 producing signals at 1550 cm-1(νa) and 1390 cm-1 (νa) of carboxylate groups. Similar changes were detected at pH 3.8 when ionic lead was present suggesting that heavy metal complexation occurs throughout a cationic exchange reaction. Vermicompost was applied to a soil where white bean plants were planted. After irrigation with lead nitrate solutions the uptake of lead was reduced to 81 % in leaves and stem, while the reduction in the roots was around 50 %. The highest accumulation of lead was found in the roots and the translocations seems to be limited by the presence off vermicompost in the soil.
Similar content being viewed by others
References
Almeida, D., Aranguren, J., Hernández, Y. and Rolo, G., (1999). Niveles de Pb en sangre de escolares. Acta Científica Venezolana, 50, 273.
Assenato G., Paci, C., Baser M. C., Molinini, R., Candela, R. M., Altamura B. M. and Giorgino, R., (1986). Sperm count suppression without endocrine dysfunction in lead-exposed men. Arch. Environ. Health, 4, 387–390.
Bellamy, L. J., (1966). The infrared spectra of complex molecules. Methuen and Co. LTD. London. 423
Boyd, S., Sommers, L. E. and Nelson, D. W., (1981). Cooper (II) and Iron (III) complexation by the carboxylate group of humic acids. Soil. Sci. Sco. Am. J., 45, 1241–1242.
Carrasquero A. and Flores, I., (2004). Cadmium bindig by humic acids. An experiment in FTIR spectroscopy and soil chemistry. Chem. Edu., 9, 1–4.
Covelo, E. F., Andrade M. L. and Vega, F. A., (2004). Heavy metal adsorption by humic humbrisols: Selectivity sequences and competitive sorption kinetics. J. Coll. and Inter. Sci., 280(1), 1–8.
Fernández, R. and Ramírez, A., (2002). Geoquímica de la contaminación urbana. Ciencia., 10(1), 94–101.
Glasstone, S., (1960). Tratado de Química Física. Ediorial Aguilar 3ra. Edición. Madrid. 1180
Ibenake, S. and Takenaka, C., (2005). Effects of dissolved organic matter on toxicity and bioavailability of copper from lettuce sprouts. Environ. Int., 31, 603–608.
Jackson, M. L., (1964). Análisis químico de suelos. Ediciones Omega, S. A. Barcelona España. 662.
Lister, S. K. and Line M. A., (2001). Potential use of sevage sludge and paper mill waste for bioadsorption of metals from polluted water ways. Bio. Tech., 79(1), 35–39.
Nakamoto, K., (1978). Infrared and raman spectra of inorganic and coordination compounds. John Wiley and Sons. New York.
Narancikova, G. and Markovnikova, J., (2003). The influence of humic acid quality on the sorption and mobility of heavy metals. Plant, Soil, Environ., 12, 565–571
Pereira, M. G. and Arruda, M. A. Z., (2003). Vermicompost as a natural adsorbent material. Characterization and potentialities for cadmium adsorption. J. Brazil Chem. Soc., 14(1), 39–47
Piccolo A. and Stevenson, F. J., (1982). Infrared spectra of Cu2+, Pb2++ and Ca2+ complexes of soil humic substances. Geoderm., 27, 195–208
Piechalak, A., Tomaszewska, B. Baralkiewicz, D. and Malecka, A., (2002). Accumulation and detoxification of lead ions in legumes. Phytochem., 60, 153–162
Rivero, C., Senesi, N., Paolini, J. and Dorazio, V., (1998) Characteristics of humic acids of some Venezuelan soils. Geoderm., 81, 227–239.
Serkar, M., Sakthi, V. and Rengaraj, S., (2004). Kinetics and equilibrium adsorption study of lead (II) onto activated carbon prepared from coconut shell. J. Coll. Inter. Sci., 279(2), 307–313.
Sparks, D. L., (1985). Soil Physical Chemistry. Delaware, USA: CRC Press. 450–487.
Sposito, G., (1986). The measurements of permanent charge. Soil Sci. Soc. Am. J., 47, 1058–1059.
Sposito, G. and Shindler, P. W., (1986). Reactions at the soil colloid-soil solution interface. Transactions of the XIII Congress of the International Society of Soil Science. Hamburg VI, 683–699.
Yilmaz, S. and Zengin, M., (2004). Monitoring environmental pollution in Erzurum by chemical analysis of Scots pine (Pinus sylvestris L.) needles. Environ. Int., 29, 1041–1047.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Durán, A.C., Flores, I., Perozo, C. et al. Immobilization of lead by a vermicompost and its effect on white bean (Vigna Sinenis var. Apure) uptake. Int. J. Environ. Sci. Technol. 3, 203–212 (2006). https://doi.org/10.1007/BF03325927
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03325927