Abstract
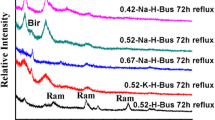
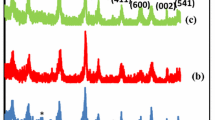
The Xiangtan manganese deposit (XTM) used to be considered a supergene oxide manganese ore in South China. We reported a new identification of the naturally outcropping cryptomelane by examining the physical, chemical and structural features of the XTM supergene oxide manganese ore. The MnO2 content was over 90%, K2O more than 3%, and water from 2.2%–3.1% which is similar to one in zeolite. The cell parameters of the cryptomelane were given as a 0 = 0.9974 nm, b 0 = 0.2863 nm, c 0 = 0.9693 nm and β = 91.47 °. There was a larger pseduotetragonal tunnel in the natural cryptomelane that was formed by [MnO6] octahedral double chains with aperture of 0.462 × 0.466 nm2, filled with K cations resulting in some Mn3+ substituting for Mn4+ to balance the negative charges of structure. The finding is important not only for prospecting manganese resources in South China, but also in application of octahedral molecular sieve of natural cryptomelane as that developed in the tetragonal molecular sieve of natural zeolite over the past century. The XTM cryptomelane (OMS-2) may be the real mineral of the active octahedral molecular sieve in nature.
Similar content being viewed by others
References
Shen, Y. F, Zerger, R. P, DeGuzman, R. N. et al., Manganese oxide octahedral molecular sieves: preparation, characterization, and applications, Science, 1993, 260: 511–515.
Ramsdell, L. S., An X-ray study of psilomelane and wad, Am. Mineral., 1932, 17: 143–149.
Richmond, W. E, Fleischer, M., Cryptomelane, A new name for the commonest of the “psilomelane” minerals, Am. Mineral., 1942, 27: 607–613.
Randall, S. R, Sherman, D. M, Ragnarsdottir, K. V., An Extended X-ray Absorption Fine Structure Spectroscopy Investigation of Cadmium Sorption on Cryptomelane (KMn8O16), Chemical Geology, 1998, 151: 95–106.
Parc, S, Nahon, D, Tardy, Y. et al., Estimated solubility products and fields of stability for cryptomelane, nsutite, birnessite, and lothiophorite based on natural lateritic weathering sequences, Am. Mineral., 1989, 74: 466–475.
Ostwald, J., Genesis and paragenesis of the tetravalent manganese oxides of the Australian continent, Econ. Geol. Bull. Soc. Econ. Geol., 1992, 87: 1237–1252.
Vasconcelos, P. M, Renne, P. R, Brimhall, G. H. et al., Direct dating of weathering phenomena by 40Ar/39Ar and K-Ar analysis of supergene K-Mn oxides, Geochim. Cosmochim. Acta, 1994, 58: 1635–1665.
Ruffet, G, Innocent, C, Michard, A. et al., A geochronological 40Ar/39Ar and 87Rb/87Sr study of K-Mn oxides from the weathering sequence of Azul, Brazil, Geochim. Cosmochim. Acta, 1996, 60: 2219–2232.
Llorca, S, Monchoux, P., Supergene cobalt minerals from New Caledonia, Canadian Mineral., 1991, 29: 149–161.
Carlos, B. A, Chipera, S. J, Bish, D. L. et al., Fracture-lining manganese oxide minerals in silicic tuff, Yucca Mountain, Nevada, USA. Chem. Geol., 1993, 107: 47–69.
Nimfopoulos, M. K, Pattrick, R.A.D., Mineralogical and textural evolution of the economic manganese mineralization in Western Rhodpe Massif, N. Greece, Mineral. Mag., 1991, 55: 423–434.
Li, J. C, Vasconcelos, P. M, Zhang, J., Behavior of argon gas release from manganese oxide minerals as revealed by 40Ar/39Ar laser incremental heating analysis, Chinese Science Bulletin, 2002, 47(18): 1502–1511.
Yao, J. Q, Wang, L. M, Su, C. G. et al., Manganese Deposits of South Marginal Zone of Yangtze Platform and Adjacent Region (in Chinese), Beijing: Metallurgical Press, 1995, 1–237.
Mukherjee, B., X-ray study of psilomelane and cryptomelane, Mineral. Mag., 1959, 32: 166–171.
Vicat, J, Fanchon, E, Strobel, P. et al., The structure of K1.33-Mn8O16 and cation odering in hollandite-type structure, Acta Cryst., 1986, B42: 162–167.
Strobel, P, Vicat, J, Qui, D. T., Thermal and physical properties of hollandite-type K1.33-Mn8O16 and (K, H3O)xMn8O16, Journal of Solid State Chemistry, 1984, 55: 67–73.
Turner, S, Buseck, P. R., Manganese oxide tunnel structure and their intergrowth, Science, 1979, 203: 456–458.
Turner, S, Buseck, P. R., Todorokites: A new family of naturally occurring manganese oxides, Science, 1981, 212: 1024–1027.
Yin, Y. G, Xu, W. Q, DeGuzman, R. et al., Studies of stability and reactivity cryptomelane-like manganese oxide octahedral molecular sieves, Inorg. Chem., 1994, 33: 4384–4389.
Luo, J., Zhang, Q. H., Huang, A. M. et al., Total oxidation of volatile organic compounds with hydrophobic cryptomelane-type octahedral molecular sieves, Microporous and Mesoporous Materials, 2000(35–36): 209–217.
Wang, Y. J, Manganese oxide ore in the 23 ° N zone in China, Collection in 80th Anniversary of the Geological Society of China (in Chinese), Beijing: Geological Publishing House, 2002, 325–329.
Editorial Committee of the Discovery History of Mineral Deposits of China, Hunan Volume, The Discovery History of Mineral Deposits of China, Hunan Volume (in Chinese), Beijing: Geological Publishing House, 1996, 1–281.
Editorial Committee of Mineral Deposits of China Mineral Deposits of China, Middle Volume (in Chinese), Beijing: Geological Publishing House, 1994, 480–552.
Post, J. E, Von Dreele, R. B, Buseck, P. R., Symmetry and cation displacements in hollandites: Structure refinements of hollandite, cryptomelane and priderite, Acta Cryst., 1982, B38: 1056–1065.
Hypolito, R, Valarelli, J. V, Giovanoli, R. et al., Gibbs free energy of formation of synthetic cryptomelane, Chimia, 1984, 38: 427–428.
Bricker, O. P., Some Stability relations in the system Mn-O-H2O at 20 °C and one atmosphere total pressure, Am. Min., 1965, 50: 1296–1354.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lu, A., Gao, X., Qin, S. et al. Cryptomelane(KxMn8−x O16): Natural active octahedral molecular sieve(OMS-2). Chin.Sci.Bull. 48, 920–923 (2003). https://doi.org/10.1007/BF03325676
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03325676