Abstract
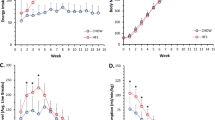
Background and aims: Moderate physical exercise and dietary restriction have both been demonstrated to delay some of the adverse effects of aging. In order to elucidate similarities or dissimilarities in their mode of action on the aging immune system in a comparative setting, we examined significant parameters of cell-mediated immunity in Sprague- Dawley rats. Methods: Male Sprague-Dawley rats, housed individually, were divided into four groups, living from 5 months (baseline group BL) up to 15, 19 and 23 months of age as follows: voluntary running in wheels (RW), food restricted by feeding to pair weight with RW animals (PW), forced running on treadmills (TM), and sedentary controls with ad libitum access to food (S1). White blood cell counts, capacity for lymphocyte proliferation in response to Concanavalin A, and interleukin-2 (IL-2) plasma concentrations were determined. Results: White blood cell counts and the cell numbers of lymphocytes, neutrophil and eosinophil granulocytes were significantly lower in the older RW and PW groups. We observed influences of forced exercise on lymphocyte proliferation: blastogenic reactivity was higher in TM animals compared with RW and PW animals at 23 months of age. Exclusively for RW animals, we found lower plasma concentrations of IL-2 at 23 months. Conclusions: Our data support the idea that moderate physical exercise modulates age-associated decline in the cell-mediated immunity of old Sprague-Dawley rats significantly more than corresponding dietary restrictions.
Similar content being viewed by others
References
Blair SN, Kampert JB, Kohl HW 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996; 276: 205–10.
McCarter RJ, Shimokawa I, Ikeno Y, et al. Physical activity as a factor in the action of dietary restriction on aging: effects in Fischer 344 rats. Aging Clin Exp Res 1997; 9: 73–9.
Viidik A, Skalicky M. Voluntary exercise and mild food restriction effectively retard the collagen biomarker of aging. Aging Clin Exp Res 2003; 15: 475–81.
McCay CM, Crolwell M, Maynard L. The effect of retarded growth upon the length of the life span and ultimate body size. J Nutr 1935; 10: 63–79.
Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 1982; 215: 1415–8.
Venkatraman JT, Fernandes G. Exercise, immunity and aging. Aging Clin Exp Res 1997; 9: 42–56.
Pahlavani MA, Cheung TH, Chesky JA, Richardson AR. Influence of exercise on the immune function of rats of various ages. J Ap- pl Physiol 1988; 64: 1997–2001.
Nasrullah I. Mazzeo RS. Age-related immunosenescence in Fischer 344 rats: influence of exercise training. J Appl Physiol 1992; 73: 1932–8.
Thoman ML, Weigle WO. Partial restoration of ConA-induced proliferation, IL-2 receptor expression, and IL-2 synthesis in aged murine lymphocytes by phorbol myristate acetate and ionomycin. Cell Immunol 1988; 114: 1–11.
Garcia GG, Miller RA. Differential tyrosine phosphorylation of zeta chain dimers in mouse CD4 T lymphocytes: effect of age. Cell Immunol 1997; 175: 51–7.
Miller RA, Stutman O. Decline, in aging mice, of the anti-2,4,6- trinitrophenyl (TNP) cytotoxic T cell response attributable to loss of Lyt-2-, interleukin 2-producing helper cell function. Eur J Immunol 1981; 11: 751–6.
Utsuyama M, Ichikawa M, Konno-Shirakawa A, Fujita Y, Hirokawa K. Retardation of the age-associated decline of immune functions in aging rats under dietary restriction and daily physical exercise. Mech Ag Dev 1996; 91: 219–28.
Tharp GD, Preuss TL. Mitogenic response of T-lymphocytes to exercise training and stress. J Appl Physiol 1991; 70: 2535–8.
Jonsdottir IH, Asea A, Hoffmann P, et al. Voluntary chronic exercise augments in vivo natural immunity in rats. J Appl Physiol 1996; 80: 1799–803.
Byun DS, Venkatraman JT, Yu BP, Fernandes G. Modulation of antioxidant activities and immune response by food restriction in aging Fisher-344 rats. Aging Clin Exp Res 1995; 7: 40–8.
Spaulding CC, Walford RL, Effros RB. The accumulation of non-replicative, non-functional, senescent T cells with age is avoided in calorically restricted mice by an enhancement of T cell apoptosis. Mech Ageing Dev 1997; 93: 25–33.
Narath E, Skalicky M, Viidik A. Voluntary and forced exercise influence the survival and body composition of ageing male rats differently. Exp Gerontol 2001; 36: 1699–711.
Novelli M, Pocai A, Skalicky M, Viidik A, Bergamini E, Masiello P. Effects of life-long exercise on circulating free fatty acids and muscle triglyceride content in ageing rats. Exp Gerontol 2004; 39: 1333–40.
Loupal G, Url A, Skalicky M, Viidik A. Physical exercise retards the development of chronic nephropathy in the ageing rat as efficiently as food restriction does. Gerontology 2005; 51: 83–93.
Strasser A, Teltscher A, May B, Sanders C, Niedermuller H. Age- associated changes in the immune system of German shepherd dogs. J Vet Med 2000; A47: 181–92.
Strasser A, Niedermuller H, Skalicky M, Hofecker G. Changes in leukocyte concentrations as biomarkers of aging in the rat. In Knook DL, Hofecker G, eds. Vienna Aging Series. Aspects of aging and disease. Wien: Facultas Verlag, 1994: 273–82.
Flaherty DK, Wagner CA, Gross CJ, Panyik MA. Aging and lymphocyte subsets in the spleen and peripheral blood of the Sprague Dawley rat. Immunopharmacol Immunotoxicol 1997; 19: 185–95.
Yano H, Kinoshita S, Kira S. Effects of acute moderate exercise on the phagocytosis of Kupffer cells in rats. Acta Physiol Scand 2004; 182: 151–60.
Gray AB, Telford RD, Collins M, Weidemann MJ. The response of leukocyte subsets and plasma hormones to interval exercise. Med Sci Sports Exerc 1993; 25: 1252–8.
Suzuki K, Nakaji S, Yamada M, et al. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc 2003; 35: 348–55.
Pahlavani MA, Vargas DA. Aging but not dietary restriction alters the activation-induced apoptosis in rat T cells. FEBS Lett 2001; 491: 114–8.
Chen J, Astle CM, Harrison DE. Delayed immune aging in diet- restricted B6CBAT6 F1 mice is associated with preservation of naive T cells. J Gerontol 1998; 53: B330–9.
Li M, Walter R, Torres C, Sierra F. Impaired signal transduction in mitogen activated rat splenic lymphocytes during aging. Mech Ageing Dev 2000; 113: 85–99.
Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev 1997; 160: 79–90.
Coleman KJ, Rager DR. Effects of voluntary exercise on immune function in rats. Physiol Behav 1993; 54: 771–4.
Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1321–9.
Riley-Roberts ML, Turner RJ, Evans PM, Merry BJ. Lympho- proliferative responses in diet-restricted and aging Sprague-Daw- ley rats. Exp Gerontol 1992; 27: 201–9.
Shephard RJ, Rhind S, Shek PN. Exercise and training: influences on cytotoxicity, interleukin 1, interleukin 2, and receptor structure. Int J Sport Med 1994; 15: 154–66.
Drela N, Kozdron E, Szczypiorski P. Moderate exercise may attenuate some aspects of immunosenescence. BMC Geriatr 2004; 4: 8.
Ginaldi L, De Martinis M, D’Ostilio A, et al. The immune system in the elderly: II. Specific cellular immunity. Immunol Res 1999; 20: 109–15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strasser, A., Skalicky, M. & Viidik, A. Impact of moderate physical exercise — in comparison with dietary restrictions — on age-associated decline in cell-mediated immunity of Sprague-Dawley rats. Aging Clin Exp Res 18, 179–186 (2006). https://doi.org/10.1007/BF03324646
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03324646