Summary
Introduction
In adults with previously untreated chronic hepatitis C (CHC), the combination of peginterferon α-2a plus ribavirin produces a higher rate of sustained virological response (SVR) than interferon α-2b plus ribavirin, but it is still unproven whether this increase is cost-effective. The objective of this study was to determine if the gain in SVR with peginterferon α-2a plus ribavirin is worth the incremental cost.
Methods
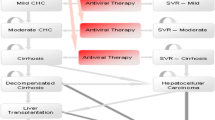
We constructed a Markov model of disease progression in which cohorts of patients received peginterferon α-2a plus ribavirin or interferon α-2b plus ribavirin for 48 weeks (hepatitis C virus [HCV] genotype 1 and non-1 patients with fibrosis) or 24 weeks (genotype non-1 patients without fibrosis), and were followed for their expected lifetimes. The reference patient was a 45-year-old male with CHC without cirrhosis. The SVRs with peginterferon α-2a plus ribavirin, and interferon α-2b plus ribavirin, were 46% and 36% for patients infected with HCV genotype 1, and 76% and 61% for patients infected with HCV non-1 genotypes, respectively. QOL and costs for each health state were based on literature estimates and on Italian treatment patterns. Costs in 2002 euros and benefits were discounted at 3%. Sensitivity analyses on key clinical and economic parameters were performed. The analysis was reported from the perspective of the Italian National Health Service.
Results
In patients infected with HCV genotype 1, peginterferon α-2a plus ribavirin increased life years (LY) by 0.78 years and quality-adjusted life years (QALY) by 0.67 years, compared with interferon α-2b and ribavirin. The incremental cost per LY and QALY gained was €9,433 and € 10,894, respectively. In patients infected with HCV non-1 genotypes, peginterferon ?-2a plus ribavirin increased LY by 1.17 and QALY by 1.01 years, compared with interferon α-2b plus ribavirin. The incremental cost per LY and QALY gained was €3,261 and €3,766, respectively. Using genotype distribution estimates, the weighted average ICER for all genotypes was €6,811 per LY gained and €7,865 per QALY gained.
Conclusion
Our model suggests that peginterferon α-2a plus ribavirin is cost-effective compared with conventional interferon α-2b plus ribavirin for treatment of naïve adults with CHC, regardless of HCV genotype, under a wide range of assumptions regarding treatment effectiveness and costs.
Similar content being viewed by others
Bibliografia
Global surveillance and control of hepatitis C: report of a WHO consultation. J Viral Hepat 1999; 6: 35–47
Mele A, Spada E. Epidemiologia delle infezioni acute da virus epatitici a transmissione parenterale. In: V Seminario di aggiornamento sull’epatite da virus HCV e nuovi virus potenzialmente epatitici: diagnosi, epidemiologia, prevenzione e terapia. Roma: ISS, Rapporti ISTISAN 00/32, 2000
Squarcione S, Carbini R, D’Amato S, et al. Epatite C in Italia nel 1992: analisi delle schede individuali. Ann Ig 1995; 7: 343–8
Farci P. Implicazioni biologiche e cliniche della quasispecie virale. In: V Seminario di aggiornamento sull’epatite da virus HCV e nuovi virus potenzialmente epatitici: diagnosi, epidemiologia, prevenzione e terapia. Roma: ISS, Rapporti ISTISAN 00/32, 2000
Ministero della Sanità — Dipartimento per la Valutazione dei Medicinali e la Farmacovigilanza. Ribavirina — interferone: una terapia per l’epatite cronica C. Boll Informazione Farmaci 2000; 3: 8–17
Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998; 352: 1426–32
Lindsay KL. Introduction to therapy of hepatitis C. Hepatology 2002; 36 (Suppl.): S114–20
McHutchinson JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998; 339(21): 1485–92
Pol S, Nalpas B, Bouriliere M, et al. Combination of ribavirin and interferon-alfa surpasses high doses of interferon-alfa alone in patients with genotype-1b-related chronic hepatitis C. Hepatology 2000; 31: 1338–44
Fried MW, Shiffnan ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975–82
Buti M, Casado MA, Fosbrook L, et al. Cost-effectiveness of combination therapy for naive patients with chronic hepatitis C. J Hepatol 2000; 33: 651–8
Wong JB, Poynard T, Ling MH, et al. Cost-effectiveness of 24 and 48 weeks of interferon alpha-2b alone or with ribavirin as initial treatment of chronic hepatitis C: International Hepatitis Interventional Therapy Group. Am J Gastroenterol 2000; 95: 1524–30
Sennfalt K, Reichard O, Hultkrantz R, et al. Cost-effectiveness of interferon alfa-2b with and without ribavirin as therapy for chronic hepatitis C in Sweden. Scand J Gastroenterol 2001; 36: 870–6
Stein K, Rosenberg W, Wong J. Cost effectiveness of combination therapy for hepatitis C: a decision analytic model. Gut 2002; 50: 253–8
Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958–65
Siebert U, Sroczynski G, Rossol S, et al. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut 2003; 53: 425–32
Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C: the OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349: 825–32
Younossi ZM, Singer ME, McHutchison JG, et al. Cost effectiveness of interferon alpha2b combined with ribavirin for the treatment of chronic hepatitis C. Hepatology 1999; 30: 1318–24
Colombo M, Sangiovanni A. Etiology, natural history and treatment of hepatocellular carcinoma. Antiviral Res 2003; 60(2): 145–50
Ministero della Sanità — Dipartimento per la Valutazione dei Medicinali e la Farmacovigilanza. Documento su: programma informazione sui farmaci, farmacovigilanza ed educazione sanitaria. Allegato 1: Epatite C — Ribavirina. 19 Nov 1999 [online]. Disponibile da URL: http://www.ministerosalute.it/medicinali/notizie/file/Informazione_farmaci.pdf [Accesso 2004 Feb 4]
Ferenci P, Shiffman ML, Fried MW, et al. Early prediction of response to 40 kDa peginterferon alfa-2a (Pegasys) plus ribavirin (RBV) in patients with chronic hepatitis C (CHC) [abstract]. Hepatology 2001; 34: 351A
Yano M, Kumada H, Kage ME, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology 1996; 23: 1334–40
Fattovich G, Giustina G, Degos F. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow up study of 384 patients. Gastroenterology 1997; 112: 463–72
Ascher NL, Lake JR, Emond J, et al. Liver transplantation for hepatitis C virus-related cirrhosis. Hepatology 1994; 20: 245–75
Detre KM, Belle SH, Lomardero M. Liver transplantation for chronic viral hepatitis. Viral Hepatitis Rev 1996; 2: 2219–28
Kilpe VE, Krakauer H, Wren RE. An analysis of liver transplantation experience from 37 transplant centers as reported to Medicare. Transplantation 1993; 56: 554–61
National Center for Health Statistics. Vital statistics of the United States, 1997. Mortality, pt A vol. 2. Washington, DC: Public Health Service, 1998
Bennett WG, Inoue Y, Beck JR, et al. Estimates of the cost-effectiveness of a single course of interferon alpha2b in patients with histologically mild chronic hepatitis C. Ann Intern Med 1997; 127: 855–65
Younossi Z, McCormick M, Boparai N, et al. Impact of chronic liver disease on patients’ utilities [abstract]. Gastroenterology 1999; 116: A1292
Kim WR, Poterucha JJ, Hermans JE, et al. Cost-effectiveness of 6 and 48 weeks of interferon-alpha therapy for chronic hepatitis C. Ann Intern Med 1997; 127: 866–74
Munari L, Picciotto A. Recombinant interferon alfa-2b therapy of chronic hepatitis C in Italy: an economic analysis. FORUM Trends Exp Clin Med 1996; 6: 347–53
Salomon JA, Weinstein MC, Hammitt JK, et al. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA 2003; 290(2): 228–37
Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001; 34: 809–16
Marcellin P, Boyer N, Gervais A, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med 1997; 127: 875–81
Lau DT-Y, Kleiner DE, Ghany MG, et al. 10-year follow-up after interferon-α therapy for chronic hepatitis C. Hepatology 1998; 28: 1121–7
McHutchison JG, Davis GL, Esteben-Mur R, et al. Durability of sustained virologic response in patients with chronic hepatitis C after treatment with interferon α-2b alone or in combination with ribavirin [abstract]. Hepatology 2001; 34: 244A
Hadziyannis SJ, Sette H Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140(5): 346–55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullivan, S.D., Craxì, A., Alberti, A. et al. Rapporto costo-efficacia della terapia peginterferone α-2a + ribavirina in confronto a interferone α-2b + ribavirina in pazienti affetti da epatite cronica di tipo C precedentemente non trattati. Pharmacoeconomics-Ital-Res-Articles 6, 105–114 (2004). https://doi.org/10.1007/BF03320628
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03320628