Abstract
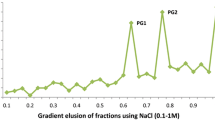
Linamarase (EC. 3.2.1.21) was purified from different tissues of cassava (leaf, rind and tuber) to compare the kinetic properties and characteristics of the enzyme in these tissues. Purified enzyme preparation appeared as single band of average molecular size 70 kD in SDS-PAGE gels. The kinetic properties of linamarase with respect to pH and temperature indicated that tuber linamarase possessed a broader pH optimum and higher temperature stability as compared to leaf and rind enzymes. Differences in Km values for linamarin were observed with leaf linamarase having the highest Km value (500 μM) followed by rind (400 μM) and then tuber (250 μM) linamarases. Rind enzyme appeared to be less susceptible to urea denaturation than the leaf enzyme. Comparison of elution profiles from DEAE-Cellulose indicated that the relative amounts of the various ionic forms of the enzyme differed in the case of each tissue. Elution patterns of the enzyme from Con A-Sepharose also differed, suggesting difference in glycosylation among leaf, rind and tuber enzymes. This was confirmed by carbohydrate analysis which showed that the tuber linamarase contained significantly higher amount of protein bound carbohydrate. These results suggest the possible occurrence of different forms of linamarase in cassava.
Similar content being viewed by others
References
Bruijn GH De, Meded Bouwhogeschool Wageningen, 71 (1971) 1.
Nambisan B & Sundaresan S, J Sci Food Agri, 66(1994) 503.
Cooke RD, Blake GG & Battershill JM, Phytochem, 17 (1978) 381.
Eksittikul T & Chulavatnatol M, Arch Biochem Biophys, 266 (1988) 263.
Yeoh MH, Phytochem, 28 (1989) 721.
Mkpong OE, Yan H, Chism G & Sayre RT, Plant Physiol, 93 (1990) 176.
Lowry OH, Rosebrough NJ, Farr AL & Randall RJ, J Biol Chem, 193 (1951) 265.
Laemmli UK, Nature, 227 (1970) 680.
Agarwal KML & Bahi OP, Methods Enzymol, 28 (1972) 720.
Nambisan B & Sundaresan S, J AOAC, 67 (1984) 641.
Ouchterlony O, Handbook on experimental immunology (1967) p 655.
Dubois M, Gilles KA, Hamilton JK, Rebers PA & Smith F, Anal Chem, 28 (1956) 350.
Hughes MA & Dunn MA, Plant Mol Biol, 1 (1982) 169.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elias, M., Nambisan, B. & Sudhakaran, P.R. Isoforms of Linamarase in Cassava (Manihot esculenta). J. Plant Biochem. Biotechnol. 6, 63–67 (1997). https://doi.org/10.1007/BF03263012
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03263012